Novel Sample Preparation and GC–MS/MS Analysis of Triclosan and Methyl Triclosan in Biosolids
Special Issues
The antimicrobial triclosan is present in myriad personal care products, many of which are disposed of down household drains and travel to wastewater treatment plants. This article describes a simple and rapid method for the preparation and extraction of triclosan and methyl triclosan from the complex matrix of biosolids and paper mulch samples followed by analysis using GC–MS/MS.
The antimicrobial triclosan is present in myriad personal care products, many of which are disposed of down household drains and travel to wastewater treatment plants. Triclosan and its metabolite, methyl triclosan, remain in biosolids even after treatment for use on agricultural lands and domestic gardening. Triclosan presents a host of environmental concerns, and methyl triclosan is assumed to have similar risks. This article describes a simple and rapid method for the preparation and extraction of triclosan and methyl triclosan from the complex matrix of biosolids and paper mulch samples followed by analysis using gas chromatography–tandem mass spectrometry (GC–MS/MS). No derivatization or cleanup steps are needed, resulting in significant time savings. Components within the paper mulch enhance the poor response of underivatized triclosan. Matrix-matched standards exploit this matrix effect and lower the limit of quantitation (LOQ) by a factor of 10. The quantitative analysis is carried out using isotopic dilution to enhance precision and accuracy. This method was used to investigate the use of earthworms (Eisenia fetida) as a bioremediation agent to remove triclosan and methyl triclosan from biosolids.
Triclosan (TCS) is one of many pharmaceutical and personal care chemicals that enter wastewater treatment plants, but are only partially removed during treatment. Some of these recalcitrant chemicals are discharged in its effluent, but others such as TCS are hydrophobic (log KOW 4.8) and primarily partition to the solids (1). Even after additional treatment, much of the TCS will remain in biosolid products destined for consumer and agricultural use.
Regardless of the treatment process used at a wastewater treatment plant or the population demographics of the source, TCS is one of the most ubiquitous identified compounds and among the highest concentration levels of analytes tested for in biosolids (2,3). TCS is also fairly stable; with a half-life of 315 to 770 days in sewage sludge and 104 days is soil after land application of biosolids (4,5). The safety of TCS in biosolids is debatable. Studies have shown that TCS has a low toxicity to plants, invertebrates, birds, and mammals and poor plant uptake, while other studies have identified effects such as endocrine disruption, algae toxicity, and possible TCS-induced antibiotic resistance (6–15). The United States Food and Drug Administration (US FDA) issued a final rule establishing that over-the-counter consumer antiseptic wash products containing certain active ingredients, including triclosan, can no longer be marketed as of September 6, 2017. The ruling was partially based on possible health risks and also on the lack of effectiveness over soap and water, but does not include consumer hand “sanitizers,” wipes, or antibacterial products used in health-care settings (16).
Methyl triclosan (M-TCS) is a metabolite of TCS that is also present in biosolids but at much lower concentrations (17). Compared to TCS, it is even more resistant to biodegradation and photolysis with a half-life of 443 days in soil after land application of biosolids (18). M-TCS is not as well studied, but it is even more hydrophobic, lipophilic, and bioaccumulative (19,20). Soil tested 4 years after biosolids application showed M-TCS had a concentration six times that of TCS, highlighting the need to understand this transformational product as much as its parent compound (21).
As they consume and degrade organic matter in soils, earthworms biomagnify inorganic and organic contaminants, including mercury, lead, cadmium, polycyclic aromatic hydrocarbons, fire retardants, pesticides, and TCS (22–26). More specifically, they can bioaccumulate chemical contaminates in biosolids amended soils (2,26,27). Kinney and colleagues (27) calculated the ratio of the concentration of contaminant inside the earthworm to the concentration of contaminant in the soil or bioaccumulation factor (BAF). Of all the contaminants detected in the worm and soil samples, TCS had the highest BAF of 27 (24). Bioaccumulation of M-TCS in earthworms has also been shown to occur in soils after biosolid applications (21).
Vermiculture is the practice of cultivating earthworms while transforming solid waste and organic biosolids into a soil amendment product. This practice can save energy, water, and reduce greenhouse gas emissions (28). Large-scale operations are currently practiced in Canada, Italy, Japan, Malaysia, the Philippines, and the United States (29,30). However, their ability to bioaccumulate pharmaceutical and personal care products (PPCPs) from biosolids before being applied to gardens, lawns, and agricultural lands remains largely unexplored and another possible benefit.
The biosolids department of Tacoma, Washington, teamed up with graduate student Whitney P. Weibel to better understand vermiculture possibilities with PPCPs. In support of her master’s thesis, “Examining the Feasibility of Vermicomposting Biosolids Prior to Land Application to Remove Triclosan and Methyl Triclosan,” the city’s environmental laboratory was tasked with developing a method for identifying and quantifying these chemicals in biosolids (31). This article takes a closer look at that research.
Method Development
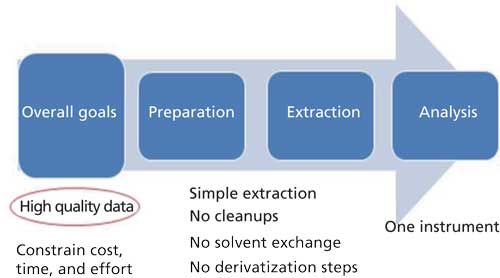
The overall goal for the laboratory was to produce reliable, useful results while constraining cost, time, and labor. Method development goals included a simple, effective extraction and analysis with minimal handling and processing required (that is, no cleanup, solvent exchange, concentration, or derivatization steps using a single injection to identify and quantify both chemicals of interest) (Figure 1).
Figure 1: Project goals.
Preparation and Extraction
The preparation and extraction is fast and simple. Worm bin samples are milled into a fine powder to enhance extraction efficiency and homogeneity, 5 mL of methylene chloride is added followed by a 2-min vortex, a 5-min sonication, another 1-min vortex, and filtration. This approach minimizes sample size, solvent usage, supply costs, and waste generated.
Analysis
M-TCS works well with gas chromatography (GC), but TCS is more suitable to liquid chromatography (LC) analysis. Many polar compounds such as TCS are better analyzed by GC with derivatization, which reduces polarity, enabling them to be eluted at reasonable temperatures without thermal decomposition while improving separation and sensitivity (32–34). Compounds like TCS that contain a functional group with an active hydrogen -OH are a major concern because of their affinity to form intermolecular hydrogen bonds (35). However, avoiding derivatization can provide better accuracy and precision by decreasing the number of sample handling steps as well as factors such as incomplete derivatizations and artifact creation, especially in crude complex matrices without cleanup (36,37).
To successfully achieve an analysis of a complex matrix without cleanup steps and derivatization, GC coupled to tandem mass spectrometry (MS/MS) was chosen. MS/MS improves selectivity and confidence in analyte confirmation while also increasing sensitivity by decreasing background interference in selected-ion monitoring (SIM) mode. Calibration standards showed very low sensitivity for TCS; however, matrix spike recoveries of the worm bin samples showed a dramatic matrix enhancement. The spike recovery immediately following the native sample analysis was above 300%, and recoveries continued to rise to nearly 600% with additional spikes in the analytical sequence before termination. M-TCS recovery also increased to 176%. This phenomenon was not seen during prior testing with 100% biosolid samples. However, the worm bin samples consisted of two components: biosolids and paper mulch. The paper mulch provides bedding and an extra carbon source for the earthworms. Each matrix component was extracted and spiked separately, and the results showed that the increased sensitivity was because of the paper mulch.
The proposed mechanism of this enhancement is called matrix-induced chromatographic response. The response improves peak intensity and shape for certain compounds in the presence of a complex matrix (38,39). The enhancement of matrix-induced chromatographic response is caused by a blockage of active sites in the injector by matrix components. Masked active sites, such as free silanol groups and metals, prevent thermal degradation-adsorption of the target analytes, thus increasing the transfer of analytes to the detector. Within the analytical column, coeluted matrix compounds that block active sites may also be responsible for the phenomenon (38). Usually, compounds prone to matrix-induced chromatographic enhancement are either thermally labile or polar analytes capable of hydrogen bonding, which is consistent with the properties of TCS. It is also a common effect observed with the analysis of certain pesticides (38,40).
Various cleanup procedures could have been used to remove the interferences and negate the enhancement; we were trying to avoid using those procedures. The alternative practice of matrix-matched calibration was chosen to compensate for matrix-induced chromatographic enhancement. Considering that the major issue with underivatized TCS is its low response, matrix-matched standards could take advantage of this matrix effect to produce higher sensitivity. As such, milled paper mulch was added to each of the calibration standards at approximately the same amount as the worm bin samples. Sensitivity rose significantly, but variability continued to be large. Before the calibration, injections of the highest concentration standard were continued until the sensitivity seemed to stabilize. However, this technique only marginally improved the variability.
Isotopically labeled internal standards were selected as a means to compensate for this variability. Theoretically, the same degree of ion enhancement should be observed for the target analytes and their isotopically labeled analogues, effectively providing a correction to account for response variability and improve the quantification accuracy (41,42). The isotopes for TCS and M-TCS were introduced before the extraction to compensate for extraction efficiency in addition to the matrix enhancement phenomenon during analysis.
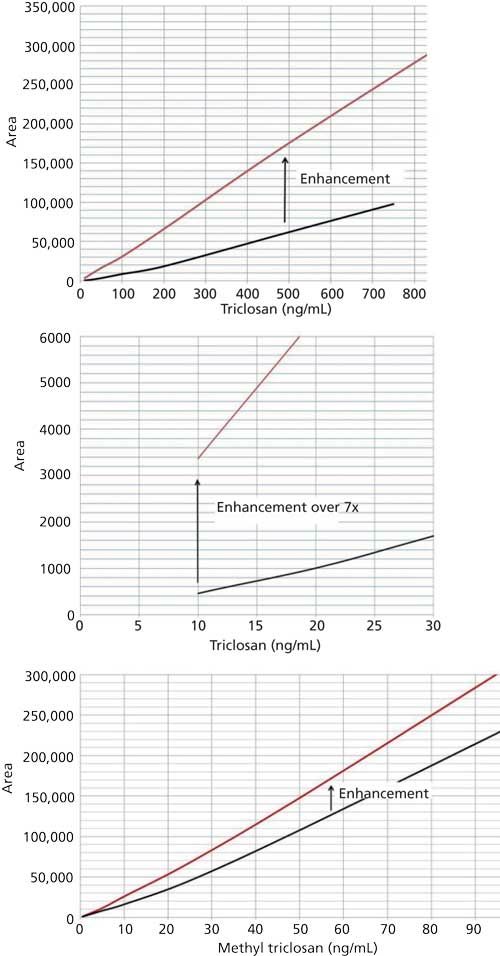
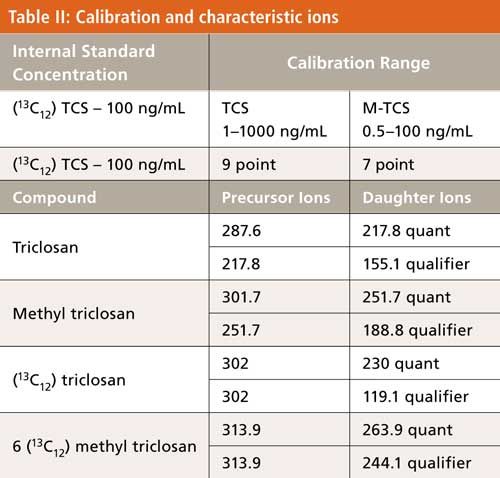
The combination of a matrix-matched calibration and isotopic internal standards proved to work well (Table I). The enhancement was significant. A greater than sevenfold signal enhancement at 10 ng/mL was observed and the low point of the linear curve decreased from 10 ng/mL to 1 ng/mL. M-TCS was also enhanced by 57% at 10 µg/mL (Figure 2). The correlation coefficients were both above 0.9996. Each calibration point was within 15% of the expected value, even at the lowest points of 1 ng/mL for TCS and 0.5 ng/mL for M-TCS. The stability of the calibration standards and instrument was periodically checked over a 35-day period. Recoveries ranged from 89% to 115% (using calibration standards ranging from 5 ng/mL to 100 ng/mL). Limits of quantitation (LOQs) of 20 ng/g for TCS and 10 ng/g for M-TCS were achieved for the method.
Figure 2: Calibration with paper mulch (top line) versus no matrix (bottom line).
Methods
TCS was purchased as part of a mix (Pharmaceutical Mix #2) from Restek and a neat M-TCS standard was purchased from Sigma-Aldrich. The 13C-labeled internal standards were purchased from Cambridge Isotope Laboratories. Trace-level methylene chloride for GC analysis was purchased from Honeywell. Paper mulch was purchased from Applegate Mulch (100% recycled newsprint without added dye).
The biosolids and paper mulch samples, free of worms and eggs, were dried in a fume hood for 96 h protected from light, milled into a very fine powder using a Retsch cryogenic mill without nitrogen cooling and stored in a freezer at -20 °C until ready for extraction. Sample extraction was performed by adding 0.25 g of sample to an 8-mL amber screw-cap vial, followed by the addition of isotopically labeled internal standards and then 5 mL of methylene chloride. The vials were vortexed for 2 min, sonicated (Branson Ultrasonic Bath 3800) for 5 min, and then vortexed again for 1 min. The supernatant was transferred to a 2.7-µm syringe prefilter (GE Whatman) and prefiltered into a 4-mL amber screw-cap vial. Then 0.5 mL of the filtrate was transferred and filtered for analysis using a 0.2-µm self-filtering autosampler vial (Agilent Technologies).
For the calibration, 0.15 g of milled paper mulch was added to each of the 8-mL amber screw-cap vials, followed by the appropriate amounts of TCS, M-TCS, and isotopically labeled standards and 5 mL of methylene chloride. The calibration standards were extracted using the same procedure as the samples. Extracts were filtered (using a 0.45-µm glass fiber filter) and the instrument was conditioned by repeatedly injecting the highest calibration standard until the area counts stabilized. This process ensured that the matrix components had sufficiently masked the active sites in the system before the calibration and samples were analyzed.
GC–MS/MS analysis was performed with an Agilent 7890 gas chromatograph equipped with a split–splitless inlet and coupled to an Agilent 7000 triple-quadrupole mass spectrometer. The split–splitless liner was a single taper 2.3-mm i.d. focus liner with inert glass wool. The inlet liner was maintained at 280 °C. A 1-µL splitless injection was used for calibration, sample, and quality control (QC) extracts. Chromatographic analysis was carried out using a 20 m x 0.18 mm, 0.18-µm df Agilent DB-5 column. Helium (purity 99.995%) was used as a carrier gas at a constant flow rate of 1.1 mL/min. The oven was programmed to start at 70 °C, held at 70 °C for 0.5 min, ramped at 10 °C/min to 178 °C, and then slowed to a ramp of 2 °C/min to 196 °C to completely resolve the closely eluted TCS and M-TCS peaks. Finally, a temperature of 320 °C was reached at a ramp of 55 °C/min where it was maintained for 3.5 min. The total analysis time was 26 min.
The MS precursor, quantitative, and qualifier ions are shown in Table II. Identification is determined by comparing GC retention time, precursor ions, and no more than a 20% difference between the ratio of qualifier to quantifier ions compared to the authentic standards. Quantitation is determined by using the response of the quantitative daughter ion (shown in Table II) and a multipoint calibration of the target analytes using the isotopic dilution technique.
Results
Much of the thesis effort was identifying suitable chemical and physical conditions for earthworm (Eisenia fetida) health and survivability in various mixes of biosolids and paper mulch (31). Unfortunately, only the repeated pilot study had earthworms survive in the material long enough for evaluation of TCS and M-TCS removal. These results showed a 75% reduction in TCS in 30 days (Figure 3). The known breakdown pathways of TCS include photolysis, chlorination, ozone treatment, and aerobic bacterial hydrolysis (43). At Tacoma’s wastewater treatment plant, chlorination does not occur until after separation of the biosolids, the substrate was mostly kept in the dark throughout the experiment, and ozone treatment is not incorporated. Aerobic bacterial hydrolysis cannot be ruled out, but only 1% of the TCS loss can be explained by the M-TCS gain (1,31). Therefore, the presence of earthworms may have had an impact on the large decrease of TCS, which is consistent with findings from previous research that earthworms can bioaccumulate TCS (2,21,27,44). However, further research is needed to fully support this proposition, including earthworm uptake studies.
Figure 3: Concentration of TCS and M-TCS before and after 30 days of vermicomposting. Adapted with permission from reference 31.
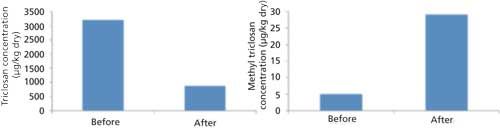
M-TCS is formed in aerobic but not anaerobic environments and through the process of microbial methylation of TCS (45). The city of Tacoma uses a brief 8–12 h aerobic digestion, followed by a 30 day anaerobic digestion, which may explain the low initial concentration of M-TCS (31). Some M-TCS was expected to form over the course of the experiment without any influence from the earthworms. Unfortunately, the repeated pilot test did not have a substrate without earthworms as a control. However, other sample sets that were terminated early had controls that indicate the M-TCS concentration increased, but less so than in the substrate with earthworms. The presence of earthworms has been shown to increase biological activity of the microbial communities within the substrate they are contained (46). This increased biological activity may be responsible for the increase in TCS breakdown while the corresponding uptake of M-TCS may occur at a slower rate. The abbreviated experimental duration is insufficient and Eisenia fetida uptake studies are needed.
The concentration of biosolids, adjusted for percent weight in the worm bin samples, ranged from 9100 ng/g to 12,800 ng/g dry, which is within literature limits. The Environmental Protection Agency (EPA) surveyed sewage sludge from 84 wastewater treatment plants subjected to secondary treatment or better and determined a concentration range of 430–133,000 ng/g dry for TCS (3). However, an LC–MS/MS screening of Tacoma’s biosolids using the same preparation and extraction procedure but with a methanol–acetone extraction mix indicated a much lower concentration of TCS, around 2200 ng/g dry (unpublished). The comparisons are from different samples at disparate sampling events and provide only a snapshot. However, efficiency between the two extractions may be a factor in the large disparity.
The method was further tested with worm bin samples containing widely different proportions of biosolids, paper mulch mix, and cake from two additional treatment plants, in Lynden, Washington, and Pierce County, Washington. Matrix spikes ranged from 83% to 143% and relative standard deviations (RSDs) were 0–3%. The increased spike recovery range may be caused by the proportion of paper mulch extracted in the calibration standards not matching all of the variations received for analysis.
Discussion and Conclusion
Using a simple extraction and a novel analysis method, excellent results can be achieved for the analysis of TCS and M-TCS in the complex matrix of biosolids and paper mulch. The preparation and extraction is fast and easy with no cleanup procedures, derivatization, concentration, or solvent exchange required. GC–MS/MS analysis involving matrix matched standards, isotopic dilution, and the use of paper mulch was used to enhance the sensitivity, selectivity, and stability of the method. The sensitivities for TCS and M-TCS were similar to other LC methods, as well as GC methods that used large-volume injections or derivatization (47–51). Lower quantitation levels were not needed for this project, but large-volume injection, a larger sample size, concentration of the extract, further optimization of the inlet, and column selection could be explored. Interestingly, M-TCS had a modest enhancement, even without an active –OH group like TCS (Figure 2).
Analyte protectants, such as D-sorbitol, gulonolactone, and pepper leaf extract, are substances added to samples and standards to block active sites within the GC system and cause enhancement in lieu of using matrix-matched standards, or when matrix-matched standards are not viable. Analyte protectants have also been used to dramatically increase the storage length and stability of organophosphorus pesticides standards (52). Their use has generally been limited to the scope of pesticide analysis. There is a dearth of research using analyte protectants in GC for other relatively polar compounds such as some PPCPs. Many phenolic compounds, such as TCS, are analyzed using LC to avoid derivatization, but some thermolabile compounds may be analyzed with adequate detection in GC using analyte protectants. Further research is needed to determine if paper mulch extract is useful as an analyte protectant for other PPCP compounds.
This work demonstrates that rather than being incinerated or dumped in landfills, biosolids can be used as a nutrient-rich soil additive. Vermiculture can potentially create a product with fewer anthropogenic chemicals before land application.
References
- M.J. Meade, R.L. Waddell, and T.M. Callahan, FEMS Microbiol. Lett. 204, 45–48 (2001).
- C.A. Kinney, E.T. Furlong, S.D. Zaugg, M.R. Burkhardt, S.L. Werner, J.D. Cahill, and G.R. Jorgensen, Environ. Sci. Technol. 40(23), 7207–7215 (2006).
- Targeted National Sewage Sludge Survey Sampling and Analysis Technical Report, January 2009, EPA-822-R-08-016.
- X. Chen, U. Pauly, S. Rehfus, and K. Bester, Chemosphere76(8), 1094–101 (2009).
- N. Lozano, C.P. Rice, M. Ramirez, and A. Torrents, Water Research47, 4519–4527 (2013).
- A.B. Dann and A. Hontela, J. Appl. Toxicol. 31(4), 285–311 (2011).
- M.E. DeLorenzo, J.M. Keller, C.D. Arthur, M.C. Finnegan, H.E. Harper, V.L. Winder, and D.L. Zdankiewicz, Environ. Toxicol. 23(2), 224–232 (2008).
- P. Fuchsman, J. Lyndall, M. Bock, D. Lauren, T. Barber, K. Leigh, E. Perruchon, and M. Capdevielle, Integr. Environ. Assess. Manage. 6(3), 405–418 (2010).
- D. Mackay and L. Barnthouse, Integr. Environ. Assess. Manage. 6(3), 390–392 (2010).
- R.S. Prosser, L. Lissemore, E. Topp, and P.K. Sibley, Environ. Toxicol. Chem. 33(5), 975–984 (2014).
- R.S. Prosser and P.K. Sibley, Environ. Int. 75, 223–233 (2015).
- R. Reiss, G. Lewis, and J. Griffin, Environ. Toxicol. Chem. 28(7), 1546–1556 (2009).
- U. S. Environmental Protection Agency (2016, July). Biosolids: Frequently asked questions about biosolids. Retrieved from https://www.epa.gov/biosolids/frequent-questions-about-biosolids
- N. Veldhoen, R.C. Skirrow, H. Osachoff, H. Wigmore, D.J. Clapson, M.P. Gunderson, G. Van Aggelen, and C.C. Helbing, Aquat. Toxicol. 80(3), 217–227 (2006).
- G.G. Ying and R.S. Kookana, Environ. Int. 33, 199–205 (2007).
- Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use. Publication Date: 09/06/2016 Food and Drug Administration Federal Register Effective Date: 09/06/2017 Document Citation:81 FR 61106 61106-61130 CFR:21 CFR 310 Agency/Docket Numbers:Docket No. FDA-1975-N-0012
- X. Chen, J.L. Nielsen, K. Furgal, Y. Liu, I.B. Lolas, and K. Bester, Chemosphere84, 452–456 (2011)
- N. Lozano, C.P. Rice, M. Ramirez, and A. Torrents, Environ. Pollut. 160, 103–108 (2012).
- M.A. Coogan, R.E. Edziyie, T.W. La Point, and B.J. Venables, Chemosphere67(10), 1911–1918 (2007).
- M.E. Balmer, T. Poiger, C. Droz, K. Romanin, P.A. Bergqvist, M.D. Müller, and H.-R. Buser, Environ. Sci. Technol. 38(2), 390–395 (2004).
- A. Macherius, D.R. Lapen, T. Reemtsma, J. Römbke, E. Topp, and A. Coors, Sci. Total Environ.472, 235–238 (2014).
- S.B. Chachina, N.A. Voronkova, and O.N. Baklanova, Procedia Eng. 113, 113–123 (2015).
- S.V. Dabke, Journal of Health and Pollution3(4), 4–10 (2013).
- C.A. Kinney, E.T. Furlong, D.W. Kolpin, S.D. Zaugg, M.R. Burkhardt, J.P. Bossio, and S.L. Werner, in Contaminants of Emerging Concern in the Environment: Ecological and Human Health Considerations (ACS symposium series, Oxford University Press, Vol. 1048, 2010), pp. 297–317.
- D. Lin, Q. Zhou, X. Xie, and Y. Liu, Chemosphere81(10), 1328–1333 (2010).
- M.W. Pannu, G.A. O’Connor, and G.S. Toor, Environ. Toxicol. Chem. 31(3), 646–653 (2012).
- C.A. Kinney, E.T. Furlong, D.W. Kolpin, M.R. Burkhardt, S.D. Zaugg, S.L. Werner, J.P. Bossio, and M. Benotti, Environ. Sci. Technol. 42(6), 1863–1870 (2008).
- R.K. Sinha, U. Patel, B.K. Soni, and Z. Li, Int. J. Environ. Waste Manage. 1(1), 011–025 (2014).
- M.K. Singh, Handbook on Vermicomposting: Requirements, Methods, Advantages and Applications (Anchor Academic Publishing, Hambury, Germany, 2014).
- A.W. Zularisam, Z. Siti Zahirah, I. Zakaria, M.M. Syukri, A. Anwar, and M. Sakinah J. Appl. Sci.10, 580–584 (2010).
- W.P. Weibel, “Examining the Feasablility of Vermicomposting Biosolids Prior to Land Application to Remove Triclosan and Methyl Triclosan,” Master Thesis, Evergreen State College, December 2016.
- J.A. Bowden, D.M. Colosi, D.C. Mora-Montero, T.J. Garrett, and R.A. Yost, J. Chromatogr. Sci.47(1), 44–51 (2009).
- D.R. Knapp, Handbook of Analytical Derivatization Reaction (Wiley & Sons, New York, New York, 1979), pp. 10, 10.
- E. Kühnel, D.D.P. Laffan, G.C. Lloyd-Jones, T. Martínez del Campo, I.R. Shepperson, and J.L. Slaughter, Angew. Chem., Int. Ed. 46(37), 7075–7078 (2007).
- V.G. Zaikin and J.M. Halket, Eur. J. Mass Spectrum. 9(5), 421–34 (2003).
- M. Mizumoto, E. Shimokita, and T. Ona, et al., J. Anal. Appl. Pyrolysis87, 163–1675 (2011).
- R.P. Evershed, in Handbook of Derivatives for Chromatography, Second Edition, K. Blau and J.M. Halket, Eds. (John Wiley & Sons Ltd., West Sussex, England, 1993), pp. 57–58.
- J. Hajslova and J. Zrostlikova, J. Chromatogr. A1000(1–2), 181–197 (2003).
- D.R. Erney, A.M. Gillespie, and D.M. Gilvydis, J. Chromatogr. A638(1), 57–63 (1993).
- F.J. Schenck and S.J. Lehotay, J. Chromatogr. A868(1), 51–61 (2000).
- B.N. Colby and M.W. McCaman, Biomed. Mass Spectrom. 6(6), 225–230 (1979).
- T. Berg, J. Chromatogr. A1218(52) 9366–9374 (2011).
- European Commission, Scientific Committee on Consumer Safety. Opinion on triclosan (antimicrobial resistance) (SCCP/1251/09). Retrieved from http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_023.pdf Luxembourg (2010).
- C.P. Higgins, Z.J. Paesani, T.E.A. Chalew, R.U. Halden, and L.S. Hundal, Environ. Toxicol. Chem.30(3), 556–563 (2011).
- W. Boehmer, H. Ruedel, A. Wenzel, and C. Schroeter-Kermani, Organohalogen Compd. 66, 1516–1521 (2004).
- J. Domínguez, M. Aira, and M. Gómez-Brandón, in Microbes at Work (Springer Berlin Heidelberg, 2010), pp. 93–114.
- B.R. Ramaswamy, G. Shanmugam, G. Velu, B. Rengarajan, and D.G. Larsson, J. Hazard. Mater. 186(2–3), 1586–1593 (2011).
- Y. Wang, P. Li, Y. Liu, B. Chen, J. Li, and X. Wang, Journal of Geoscience and Environment Protection1(2), 13–17 (2013).
- T. Anumol and S. Snyder, “Agilent High Sensitivity HPLC Analysis of Contaminants of Emerging Concern (CECs) in Water Using the Agilent 6460 Triple Quadrupole LC/MS System,” Agilent Application Note (2012).
- R.N. Hensley, J.F. Kerrigan, H. Pang, P.R. Erickson, M. Grandbois, K. McNeill, and W.A. Arnold, Environ. Sci.: Water Res. Technol. 1, 316–325 (2015).
- C.-Y. Cheng, Y.-C. Wang, and W.-H. Ding, Anal. Sci. 27(2), 197–202 (2011).
- E. Morales and A.Macherone, “Using Analyte Protectants and Solvent Selection to Maximize the Stability of Organophosphorus Pesticides during GC/MS Analysis,” Agilent Application Note (2013).
M. Bozlee is with the Center for Urban Waters, City of Tacoma Environmental Laboratory in Tacoma, Washington. Direct correspondence to: MBozlee@CityofTacoma.org

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.
Fundamentals of Benchtop GC–MS Data Analysis and Terminology
April 5th 2025In this installment, we will review the fundamental terminology and data analysis principles in benchtop GC–MS. We will compare the three modes of analysis—full scan, extracted ion chromatograms, and selected ion monitoring—and see how each is used for quantitative and quantitative analysis.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)




