Analysis of UV-Treated Mushrooms: Dietary Source of Vitamin D2?
In our study, a fast and sensitive liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS) method for the determination of vitamin D2 in fresh mushrooms and its metabolite 25(OH)D2 in the blood of volunteers regularly consuming UV-treated mushrooms has been introduced.
In recent years, dietary intake of vitamin D has become an issue of high concern because this bioactive molecule boosts the immune system and is presumed to provide some protection against Covid-19. Under these conditions, a search for nontraditional dietary sources has appeared as a new challenge. One of the possibilities is irradiation of champignons that contain a high amount of ergosterol, a vitamin D2 precursor. In our study, a fast and sensitive liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS) method for the determination of vitamin D2 in fresh mushrooms and its metabolite 25(OH)D2 in the blood of volunteers regularly consuming UV-treated mushrooms has been introduced. For extraction of desiccated mushrooms, solid-liquid extraction n-hexane–ethyl acetate was used, and n-hexane was employed for blood plasma samples. Separation of target analytes was performed on a polymeric bonding C18 phase column. Satisfactory limits of quantification (LOQs) were reached both for the control of vitamin D2 content in mushrooms (LOQ = 10 ng/g) and for the monitoring of vitamin D2 and D3 metabolite in human blood (LOQ = 2.5 ng/mL). For accurate quantification, isotopic dilution was employed.
Vitamin D plays an important role in many aspects of human health, such as skeletal and cardiovascular diseases, neuromuscular problems, diabetes, and autoimmune and chronic diseases. Today, this vitamin is also very often mentioned in connection with the support of the immune system against Covid-19 (1,2). Although vitamin D can be partially synthesized by the human body from its precursor 7-dehydrocholesterol, vitamin D deficiency is currently a global health problem (3). The level of this vitamin in the body can be increased by a diet rich in fish, eggs, milk, and dairy products. In the case of a diet suitable for vegetarians and vegans, it is possible to use UV-treated mushrooms with an increased content of vitamin D (4). Mushrooms, like human skin, can produce vitamin D when exposed to UV radiation. During this process, the content of ergosterol on the surface of the fungus is converted to ergocalciferol (vitamin D2) by a series of photochemical and thermal reactions. Thus, the amount of vitamin D2 formed in mushrooms is considerably higher than the content of vitamin D in fortified food products (5). Also, many products containing fresh or powdered mushrooms with increased vitamin D2 content are available on the retail market. Because vitamin D is a lipophilic vitamin and its excessive intake can cause problems associated with hypervitaminosis, bone loss, and kidney damage (if not treated), it is important to monitor levels of this vitamin in fortified foods (6). For this reason, it is necessary to have accurate and fast methods available for its quantification. Currently, methods used for vitamin D2 determination in mushrooms are based on solid–liquid extraction (SLE) (7–10) or saponification (11,12) of a lyophilized sample, followed by a solid-phase extraction (SPE) clean-up and determination by liquid chromatography with UV detection (LC–UV) (8,9,11) or LC–tandem mass spectrometry (MS/MS) (7,10–12). For vitamin D determination in blood plasma, methods based on protein precipitation with a subsequent liquid–liquid extraction (LLE) and LC–MS/MS determination of target analytes are usually employed (13–15). The aim of this study was to develop a fast and easy LC–MS/MS method suitable for the determination of vitamin D2 and ergosterol in fresh button mushrooms, and also a method for the analysis of the vitamin D metabolites 25(OH)D2 and 25(OH)D3 in blood plasma.
Materials and Methods
Chemicals: Acetonitrile (MeCN), methanol (MeOH), ethanol (EtOH), and ethyl acetate (EtOAc) were purchased from Honeywell. Isopropanol (iPro), n-hexane (nHex), mobile phase modifiers (ammonium formate, formic acid), ergocalciferol (vitamin D2), ergosterol, cholecalciferol (vitamin D3), 25-hydroxyergocalciferol (25(OH)D2), 25-hydroxycholecalciferol (25(OH)D3), d6-25-hydroxyergocalciferol (25(OH)D2-d6), d6-25-hydroxycholecalciferol (25(OH)D3-d6), butylhydroxytoluene (BHT), and magnesium sulfate were obtained from Merck. Hydromatrix was from Varian and anhydrous sodium sulfate was purchased from Lach-Ner. Deionized water (dH2O) was obtained from a Milli-Q Integral system (Millipore). The tested analytical columns were: 100 × 2.1 mm, 2.7-μm Poroshell 120 EC-C18 (Agilent Technologies); 100 × 2.1 mm, 1.7-μm Arion Plus C18 UHPLC (Chromservis s.r.o.); and 100 mm × 2.1 mm, 1.8-μm Zorbax Eclipse PAH RRHD (Agilent Technologies).
Sample Preparation:
Fresh Mushrooms: For the experiments and validation study, samples of fresh button mushrooms (Agaricus bisporus) and vitamin D2-enriched button mushrooms were harvested the day before the experiments were performed. Previous to the extraction, the samples were homogenized using a laboratory blender. A homogenized sample (1 g) was thoroughly grounded with 5 g of anhydrous sodium sulfate and transferred to the 50 mL PP falcon tube. Vitamin D3 standard (10 µL, 10 µg/mL) was added to the sample as an internal standard. Subsequently, 6 mL 4:6 (v/v) nHex–EtOAc extraction mixture with 0.2% BHT was added to the tube, and the tube was vigorously shaken (2 min, 1000, Geno/Grinder 2010 [Spex Sample Prep]). The upper layer was transferred into a 15-mL falcon tube and the sample was twice reextracted using 3 mL of the 4:6 (v/v) nHex–EtOAc extraction mixture. From the combined extracts, 6 mL was taken and evaporated. Subsequently, the residues were dissolved in methanol containing 0.2% BHT and transferred to the vial for LC–MS/MS analysis.
Blood Plasma: Blood plasma (0.2 mL) was transferred to the 15-mL falcon tube. A 0.02 mL (c = 500 ng/ mL) measure of 25(OH)D2-d6 and 25(OH)D3-d6 was added as an internal standard. Subsequently, 0.6 mL of MeCN with 0.2% of BHT was added for the protein precipitation. The tube was mixed using a vortex and left in a fridge to precipitate proteins (0.5 h, 4 °C). The sample was then three times reextracted using 1 mL of nHex containing 0.2% of BHT. The combined extracts were evaporated and residues were dissolved with 0.4 mL of MeOH with 0.2% of BHT.
Instrumental Analysis: For the determination of the target analytes, an Acquity UPLC system coupled to a Xevo TQS mass spectrometer (Waters) was employed. Chromatographic separation was performed using a 2.1 × 100 mm, 1.8-µm Zorbax Eclipse PAH RRHD column (Agilent Technologies). The mobile phase consisted of (A) 10 mM ammonium formate in a mixture of 1:1 (v/v) MeCN–iPro with 0.1% formic acid, and (B) 0.1% formic acid in dH2O. The elution gradient was: 0 min (85% A, 0.2 mL/min), 1 min (85% A, 0.2 mL/min), 8 min (100% A, 0.2 mL/min), 12 min (100% A, 0.2 mL/min), 13 min (85% A, 0.2 mL/min), 16 min (85% A, 0.2 mL/min). The injection volume was set to 5 µL. The mass spectrometer was operated in the positive ionization mode (ESI+) using MRM acquisition mode with the following conditions: capillary voltage, 3.5 kV; extractor voltage, 4 V; source temperature, 120 °C; desolvation temperature, 150 °C; cone gas flow, 100 L h−1; and desolvation gas flow, 1000 L h−1 (both gases were nitrogen). Argon was used as a collision gas (3.3 × 10−3 mbar). MRM transitions (collision energy) for the target analytes were: for vitamin D2 397.4>379.4 (13), 397.4>159.0 (35); for ergosterol 379.4>69.0 (18), 397.4>379.4 (13); for vitamin D3 385.4>367.2 (15), 385.4>159.0 (29); for 25(OH)D2 413.5>395.5 (10), 413.5>159.1 (25); and for 25(OH)D3 401.3>383.3 (11), 401.3>365.5 (13).
Validation Study: The recovery and repeatability of the method were tested using a vitamin D2 artificially contaminated sample (“spike”) of unirradiated fresh mushrooms at two concentration levels (50 and 200 ng/g) in six replicates. For the validation of the method for the determination of 25(OH)D2 and 25(OH)D3 in blood plasma, the standard reference material (SRM) 1950 Metabolites in Frozen Human Serum in 10 replicates and artificially contaminated sample (25(OH)D2, 5 ng/mL) of bovine serum were used.
Results and Discussion
In this study, methods for the determination of vitamin D2 in mushrooms, and 25(OH)D2 and 25(OH)D3 in blood plasma were developed. First, the LC–MS/MS method was optimized to fully separate ergocalciferol and ergosterol and to achieve a sufficient limit of quantification (LOQ) for the detection of 25(OH)D2 and 25(OH)D3 in the blood plasma. To meet these requirements, the three columns described in the “Materials and Methods” section were tested and two mobile phases consisting of MeOH–iPro–dH2O and MeCN–iPro–dH2O were tested. To improve ionization, modifiers ammonium formate and formic acid were added to the mobile phase. Two of the tested columns were commonly used C18 columns. The last one was a polymeric bonding C18 phase column that separates on the basis of shape, and this selectivity made it suitable for separating vitamin D2 and ergosterole. The best separation of ergosterol and vitamin D2 was achieved on this column in combination with a mobile phase consisting of MeCN–iPro–dH2O (see Figure 1), and therefore this column and mobile phase were used for subsequent experiments. For 25(OH)D2 and 25(OH)D3, the limits of quantification reached using this setup were 2 ng/mL resp. 1 ng/mL of blood plasma, which is sufficient to monitor these analytes in this matrix.
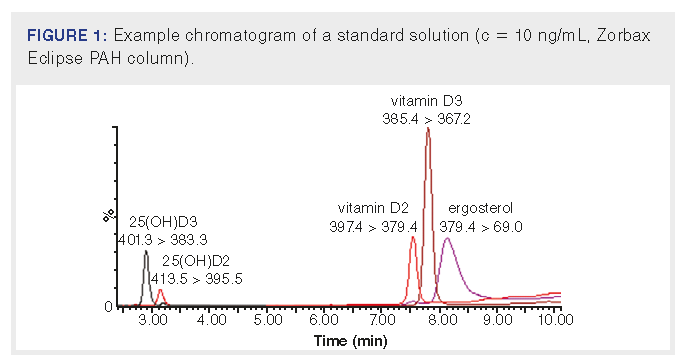
In the next step, extraction methods for vitamin D2 in mushrooms were optimized. Several parameters were tested: i) sample processing procedure before extraction, ii) the composition of the extraction solvent, and iii) the number of extraction repetitions. Mushroom sample drying before extraction is a crucial step that has a significant impact on the retention of ergosterol and vitamin D2. In most previously published studies, mushrooms were lyophilized prior to extraction, but lyophilization is a time-consuming step, and so attention has been focused on eliminating this procedure. In this experiment, three different methods of drying the sample before extraction and their effect on vitamin D2 recovery were tested: i) lyophilization, ii) drying using sorbent hydromatrix, and iii) drying using anhydrous sodium sulfate. Furthermore, fresh mushrooms were also extracted for recovery comparison. Based on the published studies, the extraction solvent nHex and solvent mixture 4:6 (v/v) nHex–EtOAc were tested. The obtained results are shown in Figure 2.
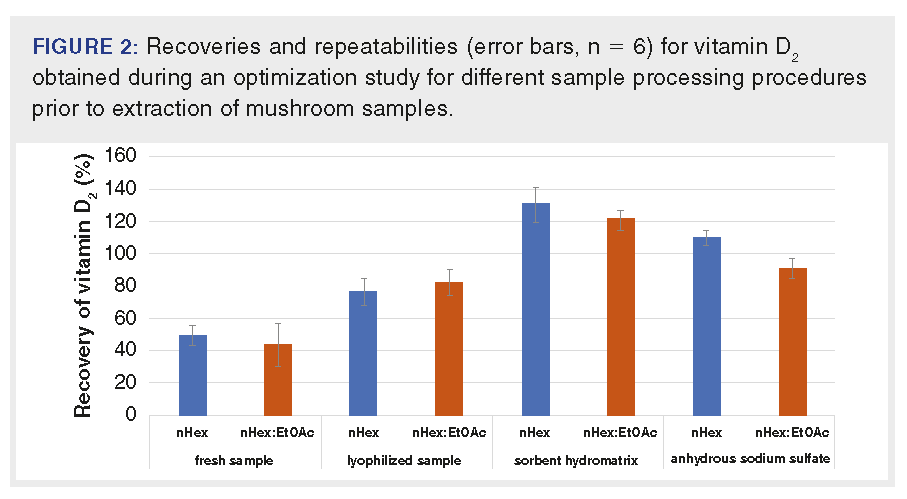
As expected, the lowest recoveries were obtained for the fresh mushroom extraction (50% and 42%) (see Figure 2). During the extraction, a gel formed in the nHex extract, which made it difficult to remove the nHex layer for the subsequent analysis, and therefore lowered the recoveries (50%). If the sample was extracted with the mixture of 4:6 (v/v) nHex–EtOAc, the gel was not formed, but the recovery of the method was also significantly reduced (42%). Sufficient recoveries for lyophilized mushroom samples (76% resp. 82%) were achieved, but as already mentioned, this method is very time-consuming and lyophilization can lead to significant losses of naturally occurring vitamin D2 (up to 16%). Therefore, this method was not selected. In case of the drying sorbents (hydromatrix and anhydrous sodium sulfate), sufficient drying of the sample as well as recoveries of the analyte of interest (130 and 110% resp. 109 and 91%) were achieved. Based on the results obtained, anhydrous sodium sulfate was chosen as the most suitable sorbent for drying of the sample before extraction due to its easy handling, availability, and price. As an extraction solvent, a mixture of 4:6 (v/v) nHex–EtOAc was selected. The last optimized parameter was the number of extraction repetitions. Usually, the vitamins are extracted from the matrix at least three times in a repeated solvent extraction, therefore three, four, and five repetitions were tested in our study. Because further extraction did not increase the recovery of the method, three repetitions of extraction were chosen as optimal.
In addition, this study also examined the method for the determination of 25(OH)D2 and 25(OH)D3 in blood plasma and optimized it. The tested parameters were the amount and type of protein precipitation solvent and the time of precipitation. In published studies dealing with the determination of vitamins in blood plasma, methanol and ethanol are most often used in a volume 2–4 times higher than the sample volume. In our study, 200 µL of blood plasma was chosen as a suitable volume of the sample and the volume of the precipitation solvent—400, 600, and 800 µL—was tested. For the precipitation solvent, methanol, ethanol, and acetonitrile were tested, and the precipitation length of 20, 30, and 40 min was assessed. The best protein precipitation occurred with the addition of 0.6 mL of acetonitrile and a precipitation time of 30 min.
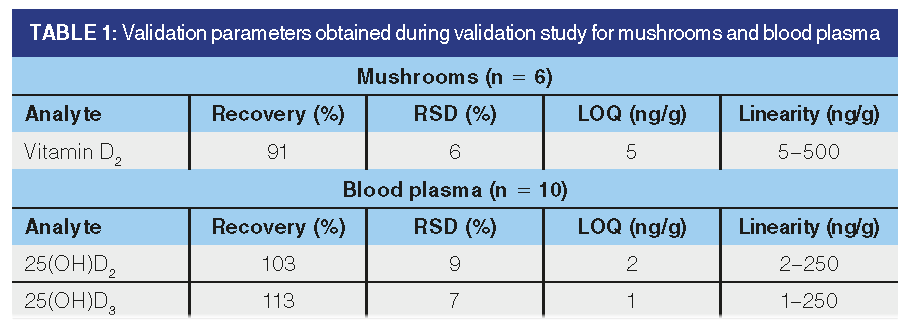
Optimized methods were subsequently validated. The obtained validation parameters are listed in Table 1. As can be seen from Table 1, satisfactory recoveries and repeatabilities were achieved for all monitored analytes, which was sufficient both for controlling vitamin D2 levels in mushrooms and mushroom products, and for the subsequent monitoring of the level of vitamin D in the blood plasma after consumption of UV-treated mushrooms.
The advantage of this optimized method over previously published studies is the speed of sample preparation, where the time-consuming lyophilization step was replaced by desiccation using a drying sorbent. The recovery of the method was further increased by the replacement of nHex extraction solvent with the solvent mixture 4:6 (v/v) nHex–EtOAc. This method for the determination of vitamin D in mushrooms can be used for fresh mushrooms to control the maximum legislative limit of vitamin D2 in irradiated mushrooms, as well as for culinary-treated mushrooms for monitoring vitamin dietary intake from mushrooms. The levels of vitamin D2 can also be monitored in the blood of consumers of UV-treated mushrooms.
Conclusion
The newly optimized method for the determination of vitamin D2 in mushrooms is based on the desiccation of fresh mushrooms using Na2SO4. The dried sample was extracted three times with 4:6 (v/v) nHex–EtOAc with 0.2% BHT. The recovery obtained in the validation study was 91%, with a repeatability of 6% and LOQ 5 ng/g. In the case of plasma, the sample was precipitated with acetonitrile with 0.2% BHT and then extracted three times with n-hexane. The method was subsequently validated and the recovery of 25(OH)D2 was 103%, with a repeatability of 9%. For 25(OH)D3, the recovery was 113%, with repeatability 7%. For separation of target analytes, a polymeric bonding C18 phase column with MeCN–Ipro was employed. In this setting, complete separation of ergosterol and ergocalciferol occurred. The LOQs obtained for all the target analytes were low enough to allow the method to be used to control the levels of vitamin D in mushrooms and mushroom products, and also to examine blood plasma samples for vitamin D metabolites.
Acknowledgement
This work was supported by the METROFOOD-CZ research infrastructure project under a grant (MEYS Grant No: LM2018100), including access to its facilities.
References
- N. Charoenngam and M.F. Holick, Nutrients 12, 2097 (2020).
- F. Mitchell, Lancet Diabetes Endocrinol. 8, P570 (2020).
- K. Amrein et al., J. Clin. Nutr. 74, 14981513 (2020).
- A. Tiwari et al., Int. J. Food Sci. Technol. 57, 1367–1377 (2022).
- J. Viraj et al., J. Food Eng. 79(3), 864–869 (2007).
- J. Janousek et al., Crit. Rev. Clin. Lab. Sci. 16, 1–38 (2022).
- J. Stevens and D. Dowell, J. AOAC Int. 95(3), 577–582 (2012).
- P. Urbain et al., Plant Foods Hum. Nutr. 71(3), 314–321 (2016).
- S. Salemi et al., LWT Food Sci. Technol. 137, 110401, (2021).
- A. Sławinska et al., Food Chemistry 199, 203–209 (2016).
- J. Ahlborn et al., J. Food Sci. Technol. 55(9), 3833–3839 (2018).
- A. Vats et al., J. Liq. Chromatogr. Relat. Technol. 44(11–12), 579–587 (2021).
- R. Rola et al., Microchem. J. 168, 106368 (2021).
- A. K. Alshabrawy et al., J. Chromatogr. B 1173, 122654 (2021).
- C. Jenkinson et al., J. Chromatogr. B 1014, 56–63 (2016).
Lucie Drábová works as a senior research assistant in the Department of Food Analysis and Nutrition, University of Chemistry and Technology, Prague (UCT Prague), Czech Republic.
Lenka Libenská is a Ph.D. student in the Department of Food Analysis and Nutrition, University of Chemistry and Technology, Prague (UCT Prague).
Markéta Zedníková is an M.Sc. student in the Department of Food Analysis and Nutrition, University of Chemistry and Technology, Prague (UCT Prague).
Veronika Vondrášková is a Ph.D. student in the Department of Food Analysis and Nutrition, University of Chemistry and Technology, Prague (UCT Prague).
Jana Hajšlová is a professor at the Department of Food Analysis and Nutrition, University of Chemistry and Technology, Prague (UCT Prague). She is head of an ISO 17025/2018 accredited laboratory and also heads a research group concerned with separation science in the fields of food, environmental, and metabolomic analysis.
Jana Pulkrabová is a professor and also Head of the Department of Food Analysis and Nutrition, University of Chemistry and Technology, Prague (UCT Prague). She is a head of the research group concerned with separation science in the fields of environmental analysis and biomonitoring.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.








