Analysis of Cellulose Molecular Weight Distributions in DMAC
The Application Notebook
SEC-MALS analysis of cellulose provides absolute molar mass distributions to understand the impact of different extraction processes. The biopolymer is solubilized in DMAC, enabling liquid chromatography without degradation.
Cellulose, a biopolymer of great importance to the fiber and paper industries, is difficult to characterize because of its high molar mass. Its intractable nature means it cannot be dissolved in conventional solvents without chemical modification. With tedious effort, it can be modified so that it can be dissolved in an easy-to-use solvent like THF, but when the cellulose is so modified it is degraded and the analysis does not represent the source material.
Unmodified cellulose can be dissolved in dimethyl acetamide (DMAC) with LiCl added. The problem remains, how to characterize it without reference to column calibration standards that typically do not have the same conformation as cellulose. Absolute characterization is performed by combining multi-angle light scattering with size exclusion chromatography (SEC-MALS) to determine molar mass, independently of elution standards.
Experimental Conditions
Separations were performed on a set of SDV-GPC columns in DMAC/LiCl. The separation columns were followed by the HPLC's UV detector, a DAWN® MALS detector (Wyatt Technology, Santa Barbara) and an Optilab® differential refractive index (dRI) detector (Wyatt Technology).
Data collection and analysis were performed in the ASTRA® software (Wyatt Technology) using empirically determined differential refractive index increments (dn/dc). Polymer molar mass M was calculated at each elution volume using signals from the two detectors.
Results
Molar masses determined by MALS in Figure 1 follow the usual logarithmic variation with elution volume. For the sake of comparison, a run of two mixed polystyrene standards is overlaid in a plot of molar mass versus elution volume. As can be clearly seen, a calibration based on polystyrene standards would overestimate the molar mass by more than a factor of five. This discrepancy is usually a result of branching, typical for cellulose in the MW range of 105–106 and above.
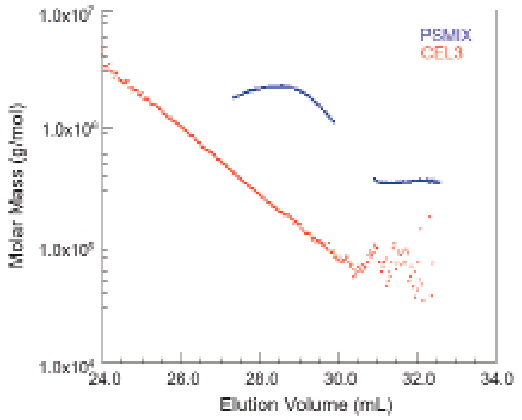
Figure 1: Two narrow polystyrene standards and a cellulose. Note that at the same elution volume, the "standard" gives a molar mass 10 times larger than the cellulose value.
The technical process of extracting the cellulose from the wood pulp can have a profound effect on the molar mass distributions. Figure 2 shows the differences in molar mass distributions arising from different extraction processes. Only a MALS detector can reveal and quantify those differences and thereby MALS has become an important tool in optimizing the production processes for cellulose.
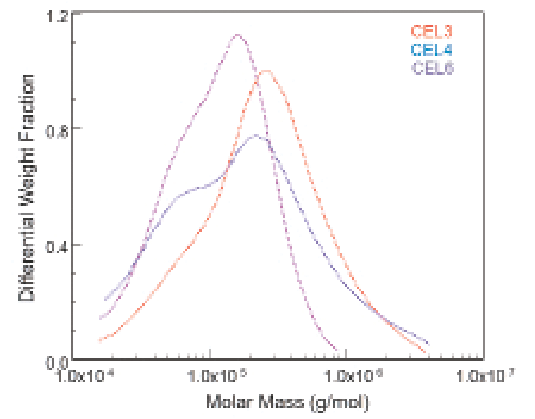
Figure 2: ASTRA's Differential Weight Distribution plot shows how different extraction processes create large variations in cellulose molar mass distributions.
Conclusions
The SEC-MALS results prove that the lengthy process of solubilizing the cellulose has been mastered, enabling the manufacturer to optimize the cellulose extraction process.

Wyatt Technology Corporation
6330 Hollister Avenue, Santa Barbara, CA 93117
tel. +1 (805) 681-9009, fax +1 (805) 681-0123,
e-mail: info@wyatt.com
Website: www.wyatt.com



















