Fast Separation of Triptans in Rat Plasma on ZirChrom®-PBD
The Application Notebook
The following reviews a published comparison of the ZirChrom®-PBD column to the Hypersil™ BDS C18 column for the analysis of triptans in rat plasma. This work concluded that the ZirChrom®-PBD phase had a superior selectivity for these analytes; allowing for an isocratic method with comparatively enhanced selectivity, peak shape and efficiency with an analysis time of less than six minutes.
Triptans are most often prescribed for the acute treatment of migraine headaches. By stimulating the brain's seratonin receptors, triptans allow the constriction of dilated blood vessels and thus alleviate pain and pressure associated with a migraine (1,2).
Traditional HPLC analysis of triptans has been complicated by the fact that they are very basic drugs. The amine moieties have a strong affinity for the silanol groups present on silica based HPLC columns causing poor peak shape, short lifetime and irreproducibility (2).
The following rapid analysis, developed and validated by Ahmed and Atia, at Taibah University (Saudi Arabia) and Assiut University (Egypt) respectively, strove to improve upon currently available methods (2). The zirconia-based ZirChrom®-PBD was chosen by the authors for its lack of silanol groups, different selectivity, and unparalleled thermal and chemical stability.
Experimental
Four triptans were analyzed: Sumatritan succinate (SMT), Zolmitriptan (ZLT), Eletriptan hydrobromide (ELT) and Rizatriptan benzoate (RZT). Standard stock solutions were prepared by dissolving the samples in pure acetonitrile to a concentration of 1 mg/mL. The samples were then diluted using the appropriate mobile phase and used to spike a sample of processed rat plasma to a final concentration of 1000 ng/mL. The following chromatographic conditions were used:

Click here to view full-size graphic
In Figure 1, the ZirChrom®-PBD clearly provided superior selectivity and was able to do so faster, more efficiently and with less organic solvent used than the silica column (2). The unique selectivity and thermal stability of the ZirChrom®-PBD phase allows for baseline resolution of the four triptans in under six min. The longer elution time and poor peak shape of the compounds on the Hypersil™ BDS C18 column was attributed to residual silanol interactions.
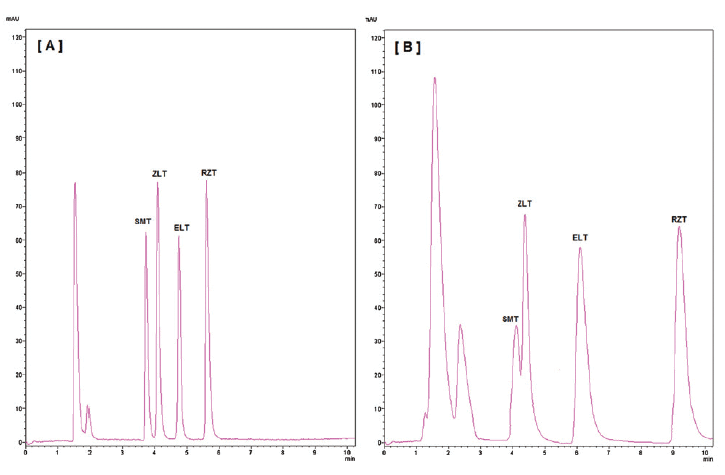
Figure 1: Comparison of (A) ZirChrom®-PBD and (B) Hypersil™ BDS C18 for the analysis of four triptans. Used with permission (2).
The efficiency (N - theoretical plates) on ZirChrom®-PBD improved for all and for three of the compounds the improvement was ten-fold (2). When validating this method on ZirChrom®-PBD the authors found that the ZirChrom®-PBD column had a wider calibration range and improved sensitivity when compared to other HPLC and UV methods (2).
References
(1) H.C. Diener, H. Kaube, J. Neurol.,246, 515–519 (1999).
(2) S. Ahmed, N.N. Atia, J. Pharm. Biomed. Anal.,143, 241–251 (2017).
Hypersil™ BDS C18 is a registered trademark of Thermo Fisher Scientific™

ZirChrom Separations, Inc.
617 Pierce Street, Anoka, MN 55303
tel. 1 (866) STABLE-1
Website: www.zirchrom.com














