RPC-MALS Analysis of Protein Oligomers
The Application Notebook
Reverse-phase chromatography can be combined with multi-angle light scattering to separate and characterize protein oligomers and isoforms not resolvable by size-exclusion chromatography.
Reversed-phase chromatography (RPC) represents one of the most popular applications of HPLC, with particular importance for protein characterization. Because elution depends on the hydrophobicity of the sample, it is generally impossible to identify the separated products on the basis of their elution time (volume). Frequently, each eluted fraction must be isolated further and analyzed with other techniques in order to gain some understanding of the molecular behavior of the protein.
Adding a miniDAWN® or DAWN® multi-angle light scattering (MALS) detector to one's HPLC system, however, simplifies protein identification significantly. It allows one to measure absolute molar masses directly, irrespective of retention time, and to observe the properties of the protein in solution.
Experimental
The experimental system used to collect the data shown here consisted of a miniDAWN connected downstream of an HPLC quaternary pump, degasser, autoinjector, and UV diode-array detector. A Vydac Protein C-4 column (150 mm × 4.6 mm, 300 Å) was used with a flow rate of 0.7 mL/min. ASTRA® software collected and analyzed the data to determine absolute molar mass and size.
Results
The inset of Figure 1 shows a chromatogram of basic fibroblast growth factor (bFGF) with a particular degree of oxidation produced by 0.2 equivalents of DTNB (1). The light scattering signal for each of the first two peaks is approximately twice the UV signal, while for the third peak the two signals are equal. This shows at a glance that the three peaks correspond, from left to right, to two types of dimers and a single monomer of bFGF; calculations show the molar masses to be 33 kDa, 34 kDa, and 17 kDa, respectively.
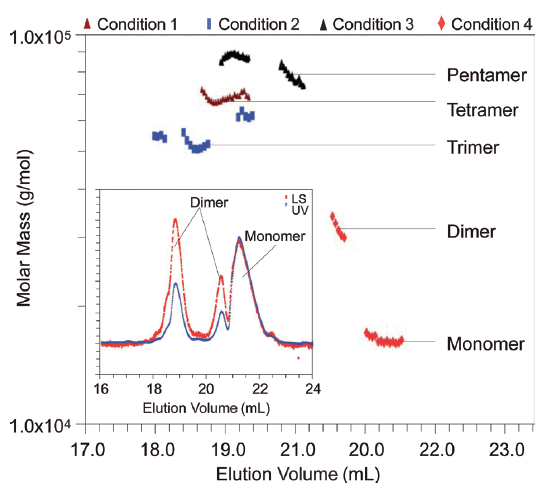
Figure 1. Inset: UV signal of basic Fibroblast Growth Factor (bFGF) with 90° light scattering (LS) signal corrected for gradient baseline shift. Main: Absolute molar masses at the peak regions for bFGF prepared under four different oxidation conditions.
The main part of Figure 1 presents a superposition of molar mass versus elution volume from four different separations, each based on a different degree of oxidation. The presence of dimers, trimers, tetramers, etc. is clearly evident, yet there is no consistency in the order of the elution volume.
MALS measurements made with the miniDAWN permit the absolute identification of each eluting peak. In addition, the root-mean-square radius of an eluting molecule may often be determined. For example, the rms radius of the bFGF pentamer was shown to be about 13 nm, indicative of a rod-like structure for this 85 kDa multimer.
Conclusions
Simply adding a miniDAWN to RPC permits the absolute determination of the molar mass of each eluting fraction, the detection and identification of different multimeric forms, and the size and conformation of the separated molecules in solution.
References
(1) I. V. Astafieva, G.A. Eberlein, and Y.J. Wang, J. Chromat. A 740(2), 215–229 (1996).

Wyatt Technology Corp.,
6330 Hollister Ave., Santa Barbara, CA USA 93117
tel. 1 (805)681-9009, fax 1 (805)681-0123
Website: www.wyatt.com














