Advances in Supercritical Fluid Chromatography for the Analysis of Chiral and Achiral Pharmaceuticals
Special Issues
This article provides an overview of the most recent advances in the field of chiral and achiral separations in SFC. This involves research focused on the most critical parameters in SFC separations, but also on practical issues such as the serial coupling of columns.
A revival of supercritical fluid chromatography (SFC) has been observed recently. SFC has repeatedly enabled fast and efficient separations, and in some cases has even outperformed high performance liquid chromatography (HPLC). This article provides an overview of the most recent advances in the field of chiral and achiral separations in SFC. This involves research focused on the most critical parameters in SFC separations, but also on practical issues such as the serial coupling of columns. The recent evolution from classic SFC to ultrahighperformance supercritical fluid chromatography (UPSFC) is also discussed.
In 1962 Klesper et al. (1) were the first to report supercritical fluids as eluents in chromatographic separations. A supercritical fluid is obtained by elevating the temperature and pressure above the characteristic critical values of the substance concerned. This reversible physical state possesses unique and interesting properties that can be applied in chromatography. Because the viscosity and diffusivity of this phase are comparable to that of gaseous mobile phases, lower pressures are generated over the system and column. The density and solvating power, on the other hand, approach that of liquid mobile phases, creating a broad application range.
However, having to compete with other popular techniques, such as high performance liquid chromatography (HPLC) and gas chromatography (GC), the interest in supercritical fluid chromatography (SFC) was rather limited in the past and applications were relatively scarce. Additionally, SFC users pushed against the limitations of the available instrumentation to apply this technique. A strict control of the mobile phase density was vital to allow accurate and reproducible analyses, but often quite tedious to achieve. In addition, conventional ultraviolet (UV) detection was complicated by the supercritical state of the mobile phase. The refractive index of this phase is directly proportional to its density. Consequently, small changes in mobile phase density can drastically influence the refractive index and thus impede the UV-detection. Inspired by the technological evolution of HPLC, SFC has undergone major instrumental improvements over recent years. This has led to a revival of this technology, which is now routinely applied in a number of pharmaceutical laboratories. Inspired by the technological evolution of HPLC, SFC has undergone major instrumental improvements over recent years. The mechanisms of the back pressure regulator and oven have been improved, allowing a much stricter control of the mobile phase density. The system's void volume has been reduced, resulting in higher separation efficiencies, and adjustments to the flow path of the UV detector has resulted in an improvement of the baseline noise. This has led to a revival of this technology, which is now routinely applied in a number of laboratories. In this overview, the most recent advances and applications in SFC will be highlighted, with a special focus on its application in pharmaceutical separations.
Supercritical Fluid Technology
Temperature and Pressure: First of all, the term supercritical fluid chromatography should be taken into consideration. Carbon dioxide is used almost exclusively as the main eluent in SFC and turns into a supercritical state above approximately 31 °C and 73 bar. Because the polarity of pure carbon dioxide is too low to allow analysis of more polar compounds, the addition of polar organic modifiers (OM) to the mobile phase is often essential. The critical point of the resulting mobile phase is therefore increased to higher temperatures and pressures. With usual concentrations of OM between 5–35%, the vast majority of SFC analyses are therefore actually performed at subcritical conditions. This does not pose any issue, because the properties of the supercritical state also apply to the subcritical one (2). However, most SFC users tend to work around the theoretical supercritical point of pure carbon dioxide, rather than deviating further into the subcritical region. Instrumental constraints limit users to pressures and temperatures below 400 bar and 50 °C, respectively, while the stationary phase limitations are even stricter. Consequently, the usable region for explicit supercritical fluid separations is rather restricted (3).
The selectivity of SFC separations is often optimized by altering the OM type or fraction in the mobile phase, in analogy with conventional HPLC method development. Although the effects of temperature and pressure on the mobile phase density and selectivity are wellknown, these parameters are often chosen empirically and are not thoroughly investigated.
More recently, there has been a tendency to investigate effects of temperature and pressure more extensively. Reports were made of SFC analyses performed at sub-ambient temperatures and back pressures below 150 bar, thus further exploring the subcritical region (Figure 1). In theory, the lowest operable pressure at a given temperature in SFC is therefore not the critical pressure of CO2, but rather the pressure at which the transition from a liquid to vapour phase occurs at the same temperature, which is much lower. Tarafder and Guiochon (3) recently published a paper on the potential advantages of analyses in this subcritical region. By lowering the back pressure, the allowable pressure drop over the column increases. Since the pressure drop is directly proportional to the product of the flow rate and column length, an increase in pressure drop enables increased flow rates, which reduces the analysis times, and longer column lengths, which improves efficiency. However, applying higher flow rates may have a negative influence on the efficiency and thus there are limitations to how much the flow rate can be elevated. Moreover, the column stability needs to be taken into consideration in terms of the maximum allowable pressure drop. Research also showed that a broader temperature range can be explored to alter the selectivity, starting from temperatures as low as 0 °C up to the instrument or stationary phase limitations (3,4). However, the majority of current SFC instruments are not properly equipped to perform analyses at these operating conditions.
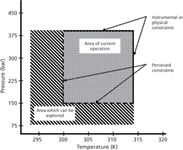
Figure 1: Schematic representation of the different regions in which supercritical fluid chromatography (SFC) is mostly employed, more specifically the sub- and supercritical state. Adapted and reprinted from J. Chromatogr. A 1265, A. Tarafder and G. Guiochon, Extended zones of operations in supercritical fluid chromatography, 165â175 (2012) with permission from Elsevier (3).
Mobile Phase Additives in SFC: In SFC, mobile phase additives are often necessary to achieve satisfactory chromatographic results. Residual silanol groups interact strongly with ionizable functional groups of molecules, such as amines, potentially resulting in distorted peak shapes. Acidic functional groups might prevent elution and result in excessive retention. To overcome these deleterious effects, polar additives can be dissolved in the mobile phase, usually at concentrations between 0.1–2.0%. In general, acidic additives such as trifluoroacetic acid (TFA), acetic acid and formic acid (FA) are used when analysing acidic compounds, while basic amine additives such as diethylamine (DEA), triethylamine (TEA) and dimethylethylamine (DMEA), are applied for basic compounds. They exert an effect through multiple interaction mechanisms with the analyte, depending on its nature. Both basic and acidic additives also decrease aspecific interactions with the silica matrix. CO2 displays an acidic character in the presence of protic organic modifiers, such as methanol. As a result, interaction can occur between this main mobile phase substituent in SFC and analytes with basic functional groups. Through the above mechanisms, additives can alter the selectivity (5). As a result, a great deal of interest in SFC method development was paid to the use of additives.
Recently, ammonium hydroxide has been used as a mobile-phase additive for preparative separations. Because NH3 (ammonia) is volatile at ambient conditions, additive removal after the separation can simply be achieved by reducing the pressure. Hamman et al. (6) demonstrated the column stability and chromatographic efficiency when using aqueous ammonia as a mobile phase additive. Later, Ventura et al. (7) investigated the use of methanolic ammonia for chiral and achiral separations in an attempt to avoid the introduction of water into the SFC equipment, which can potentially deteriorate the chromatographic results. They showed a similar effect of ammonia compared to DEA and DMEA on the efficiency in terms of retention and selectivity and also claimed an increase in mass spectral sensitivity.
The combined use of an acidic and a basic additive in SFC has also been investigated in the context of chiral separations (8). It was shown that by using isopropylamine and TFA together in the mobile phase, a different enantioselectivity is generated compared with the separate additives. The combination of additives can be used for the separation of all types of compounds regardless of their acid–basic properties.
Tandem-Column Coupling in SFC
The low pressure drops generated in SFC enable the tandem coupling of columns. With this approach, selectivity or efficiency can be altered by serially coupling different or identical columns, respectively. This can be particularly interesting for chiral columns, which usually display only a limited achiral selectivity and a relatively low efficiency. Most chiral samples encountered in the pharmaceutical domain consist of multiple stereoisomers or of compounds in a complex matrix together with impurities and excipients. By coupling chiral and achiral stationary phases, the separation of these complex samples can be achieved (9). An important requirement when coupling (a)chiral columns is their compatibility in relation to the mobile phase. As both reversed-phase LC and normal-phase LC columns can be used in SFC, this poses no limitations in practice. When coupling columns in SFC, back pressure depends on their position in the instrument, which implies that chromatographic behaviour can differ significantly when switching the column order (10).
Phinney et al. (9) successfully separated four racemic β-blockers by coupling an achiral 25-cm cyanobonded phase (J.T. Baker) and a 25-cm Chiralcel OD-H (Chiral Technologies). They also separated a mixture of eight benzodiazepines by tandem-coupling an amino-bonded phase to Chiralcel OD-H (Chiral Technologies). When coupling an achiral and a chiral column, the total retention of compounds is equal to the sum of the retention on the individual columns. Thus, the net retention can be estimated and suitable column combinations can be proposed, based on information from individual column screenings.
Brunelli et al. (11) serially coupled five 25-cm cyanopropyl silica columns to achieve higher plate counts. This enabled them to achieve the achiral separation of a complex mixture of 17 pharmaceutical compounds with a high resolution in 20 min. They also noticed that the effect of the temperature on the efficiency and selectivity increases significantly when coupling columns. Barnhart et al. (12) developed a separation method for a mixture of four unnamed stereo-isomers by coupling two 15-cm columns, for example, Chiralpak AD-H and Chiralcel OD-H (Chiral Technologies). A successful upscaling of this method was done to preparative scale with a flow rate of 55 mL/min.
Welch et al. (10) developed an automated tandem-column screening tool for chiral separations by modifying a commercially available analytical SFC instrument. Their setup allowed 10 single columns and up to 25 different single-column arrangements to be screened. Three basic strategies can be employed: (a) coupling a chiral and an achiral column; (b) coupling two identical chiral columns; and (c) coupling two different chiral columns. They used the adapted equipment for the development of a method to separate a four-compound stereoisomeric mixture of in-housecoupled racemic ibuprofen with racemic 1-phenylethylamine. Coupling a chiral and achiral column often failed to provide the necessary selectivity, because of the poor stereoselectivity of achiral stationary phases. The setup with two different chiral columns provided the best results. This approach offers the potential for a wide range of selectivity to be achieved. However, an empirical screening of the coupled columns is required, since the retention on two coupled chiral columns cannot be estimated as the sum of retentions on the individual columns. Figure 2 presents the method screening and development of the separation of four stereoisomers.

Figure 2: Automated screening and method development of racemic ibuprofen coupled with racemic 1-phenylethylamine by tandem coupling of two chiral stationary phases. The standard gradient method entails: isocratic carbon dioxide/methanol, 96/4, v/v, for 4 min, then ramp at 2%/min to 40% methanol, 1.5 mL/min, 200 bar, 35 °C, UV 215 nm, 25 min run time. Adapted and reprinted from Chirality 19(5), C.J. Welch, M. Biba, J.R. Gouker, G. Kath, P. Augustine and P. Hosek, Solving multicomponent chiral separation challenges using a new SFC tandem column screening tool, 184â189 (2007) with permission from John Wiley and Sons (10).
Ultrahigh Performance SFC
Sub-2-µm Particles: Because the mobile phase viscosity is directly proportional to the generated pressure drop across a column, sub-2 µm particles have great potential in SFC, decreasing analysis times and increasing efficiency. However, very little research has been conducted with this type of stationary phase in SFC. Berger (13) investigated and demonstrated the applicability of 1.8-µm particles in SFC. Highly efficient achiral separations below 1 min of a diverse range of compounds, for example steroids, sulphonamides, profens, are presented in his paper. He also noticed significantly lower inlet pressures and pressure drops compared to HPLC separations. Using flow rates of 2 mL/min, pressure drops below 100 bar were typically generated, compared to the average of 1000 bar back pressure generated in UHPLC mode. Thus, these sub-2-µm particles are very suitable for routine use in SFC to improve efficiency and analysis times in a straightforward manner.
For chiral separations, no sub-2-µm stationary phases are commercially available. It appears to be difficult to convert these small particles into chiral stationary phases (CSPs), with an acceptable batch-to-batch reproducibility. For chiral separations, users are therefore compelled to use conventional 5 µm or 3 µm CSPs, with lower efficiencies.
Inspired by the advances made over recent years in the field of UHPLC, major instrumental improvements led to the introduction of an ultrahigh performance SFC (UPSFC) equipment by Waters. The system is optimized specifically for SFC with very low void-volumes, improved detector, flow cell, pump modules and back pressure regulator. This equipment claims to increase the throughput and efficiency of analyses, especially when used in combination with achiral sub2-µm particles. However, since the introduction of this equipment is very recent (2012), literature references are still limited to application notes and its actual performance in routine analysis has yet to be confirmed (14).
Porous-Shell Particles: Porous-shell particles have rapidly found acceptance in the field of HPLC, given their applicability with conventional and readily available HPLC equipment. Because the diameter of these particles is 2.6 µm, a lower pressure drop will be generated compared to sub-2µm columns. The hard non-porous centres of the particles are coated with a layer of totally porous silica, allowing high efficiencies in terms of analysis times and plate counts to be obtained. These porous-shell particles outperformed sub-2µm particles in UHPLC (15). Berger (16) investigated the applicability of these 2.6-µm porous-shell particles in achiral SFC. The separation of a 17 compound achiral test mix was compared using a fused-core column (Kinetex HILIC, Phenomenex) and a 3-µm fully-porous bare silica column (Luna silica, Phenomenex) (Figure 3). Results showed that the porous-shell particles also outperformed the totally porous particles in SFC, as in UHPLC. Lower theoretical plate heights, significantly shorter analysis times, better resolutions and lower pressure drops were obtained. However, many peaks displayed peak fronting of which the source is not yet determined. Porous-shell particles have great potential in SFC, obtaining higher efficiencies in shorter time spans. Up till now, only achiral stationary phases are commercially available with this type of particle. As chiral separations often suffer from low efficiencies in terms of plate count, these porous-shell particles could indicate a serious improvement. Up until now, to our knowledge, they were only tested in LC and capillary electrochromatography (CEC) settings (17,18).

Figure 3: Separation of a 17 component mixture. (a): fused-core 4.6 à 150 mm, 2.6 µm column, (b): fully porous 4.6 à 150 mm, 3 µm bare silica column. Supercritical fluid chromatography conditions: 3.5 mL/min, 15% methanol in CO2, 175 bar back pressure, 50 °C. Adapted and reprinted from J. Chromatogr. A 1218(28), T.A. Berger, Characterization of a 2.6µm Kinetex porous shell hydrophilic interaction liquid chromatography column in supercritical fluid chromatography with a comparison to 3µm totally porous silica, 4559â4568 (2011) with permission from Elsevier (16).
Chiral SFC Applications
SFC is especially suitable to achieve enantioseparations on CSPs. Numerous examples of SFC enantioseparations have been published (5,19,20). In this area, the polysaccharide-based stationary phases have undoubtedly conquered a dominant position, because of their easy accessibility and proven broad enantioselectivity. Although chiral columns dedicated to SFC applications are being introduced nto the market, any (normal-phase or reversed-phase) HPLC CSP can be employed in practice. Recently, a focus is being placed on the immobilization of the chiral selector to the silica matrix. Although these columns still have limitations in terms of maximum allowable pressure, the compatibility with solvents that dissolve the coated selector of classic CSPs is greatly extended. In addition, it is claimed that these immobilized CSPs have a longer column life time. Table 1 provides an overview of the recently introduced immobilized CSPs. However, only a few reports can be found in the literature concerning the performance of these columns in SFC, as most research is still being conducted in HPLC. In this latter technique, it has been noticed by several authors that CSPs with the same chiral selector, either coated or immobilized, on the silica matrix, yield a different enantioselectivity under the same conditions. The immobilization alters the higher-order structure and configuration of the polysaccharide-based selector and increases its rigidity, which can be at the expense of the enantioselective recognition (21).

Table 1: Commercialized chiral stationary phases with an immobilized chiral selector.
Miller (21) investigated the immobilized columns IA, IB and IC (Chiral Technologies) in SFC using mixtures of methanol and nontraditional modifiers, for example, dichloromethane and tetrahydrofurane, for enantioseparations. These nontraditional mixtures may offer a solution for the separation of racemates with poor methanol solubility. A drastic difference in enantioselectivity was reported using traditional compared with non-traditional modifiers. The rate of unresolved racemates was mostly higher using non-traditional modifiers on the immobilized columns. Based on these results it was advised not to include these modifiers in a primary screening approach, but rather to test them for compounds not resolved with the conventional OM. Further research in SFC concerning the similarity of coated and immobilized CSPs with the same selector was not found in the literature.
Achiral SFC Applications
A major part of the reported applications with SFC concern chiral separations. As conventional HPLC offers a suitable solution for most achiral separations, users are not inclined to switch to SFC, which is often perceived as a more complex version of HPLC.
In theory, both normal- and reversed-phase stationary phases (SPs) can be used in SFC. However, in recent applications there seems to be a preference for polar stationary phases (normal phase). This choice is based on the relatively apolar nature of the major constituent of the mobile phase (carbon dioxide). Frequently used SPs are based on bare silica or silica derived with amino-, cyanopropyl- or ethylpyridine groups (22). However, since any HPLC phase can be employed, the range of useful columns can be extended far beyond this list, for example, SP based on silica with cross-linked diol groups are used to perform SFC in hydrophilic interaction chromatography (HILIC) mode, or cation-exchange SP can be used in ion-exchange modes (22,23).
West et al. (23) investigated 11 HILICtype SPs in SFC with 146 test compounds and illustrated their applicability with the analysis of three mixtures of drug-like compounds. The research indicated that silica phases are the most acidic and therefore provide the strongest interaction with basic solutes. Nitrogen-containing SPs interact the strongest with acidic analytes given their strong basic nature. An intermediate behaviour is displayed by neutral SPs, for example, amide and alcohol types. Consequently, an appropriate column type can be chosen depending on the sample nature.
Recently, the applicability of SFC in the field of achiral separations has been extended by the introduction of commercial SFC–MS equipment. However, finding the appropriate generic stationary phase is the main constraint in achiral purifications. De la Puente et al. (24) defined and implemented a generic screening strategy for the method development of separations of achiral mixtures in SFC–MS. Screening five complementary SPs — diol, 2-ethylpyridine, diethylaminopropyl, benzenesulphonamide and dinitrophenyl — in a five min gradient with a mobile phase containing methanol was proposed. An exemplary result of their screening method applied on an in-house mixture is presented in Figure 4. This screening was routinely applied for three years and achieved overall success rates >85%. Therefore, the implementation of their screening was successful in terms of efficiency and cost-effectiveness.

Figure 4: Chromatograms of the screening approach on an in-house mixture of three compounds, illustrating the complementarities of the five different stationary phases. Adapted and reprinted from J. Chromatogr. A 1250, C.F. Poole, Stationary phases for packed-column supercritical fluid chromatography, 157â171 (2012) with permission from Elsevier (22).
Conclusion
After overcoming the initial problems with SFC, a revival of this technique has been seen over recent years. In general, the efficiency, selectivity and performance of the technique has been investigated and attempts made to improve these aspects from various angles. Attention is being paid to the influence of the most critical parameters: temperature and pressure. More specifically, lower temperature and pressure ranges are being explored, and aim to achieve different selectivities. The same is aimed for using higher flow rates. Additives also play an important role in SFC separations, and in this context the applicability of different additive types has been investigated. Tandem column coupling has also been researched recently. As lower pressures are generated in SFC, this technique lends itself perfectly for this purpose. By serially coupling identical or different columns, higher plate counts or unique selectivities can be achieved, respectively. Finally, ultrahigh-performance SFC is emerging. Following the example of HPLC and UHPLC, ultrahigh-performance SFC equipment has been commercialized in 2012 and the use of sub-2-µm and porous-shell particles is gaining attention. Meanwhile, more and more research is demonstrating the performance and applicability of SFC for chiral and achiral separations. SFC is still behind LC in the literature, but it could well deserve an equal position.
Yvan Vander Heyden is a professor at the Vrije Universiteit Brussel, Belgium, Department of Analytical Chemistry and Pharmaceutical Technology, and heads a research group on chemometrics and separation science.
Debby Mangelings is also a professor at the Vrije Universiteit Brussel, Belgium, Department of Analytical Chemistry and Pharmaceutical Technology. Her research concerns a.o. chiral separations with different separation techniques.
Katrijn De Klerck is a Ph.D. student in the Department of Analytical Chemistry and Pharmaceutical Technology. Her main research area focuses on the evaluation of polysaccharide-based stationary phases in supercritical fluid chromatography and the application of chemometric techniques to chromatographic data.
References
(1) E. Klesper, A.H. Corwin and D.A. Turner, J. Org. Chem. 27(2), 700–706 (1962) .
(2) K.D. Bartle, in R.M.Smith (Editor), Supercritical fluid chromatography, Royal society of chemistry, Loughborough, UK, pp 1 (1988).
(3) A. Tarafder and G. Guiochon, J. Chromatogr. A 1265, 165–175 (2012).
(4) J. Zauner, R. Lusk, S. Koski and D.P. Poe, J. Chromatogr. A, 1266, 149–157 (2012) .
(5) K. De Klerck, D. Mangelings and Y. Vander Heyden, J. Pharm. Biomed. Anal., 69, 77–92 (2012).
(6) C. Hamman, D.E. Schmidt Jr., M. Wong and M. Hayes, J. Chromatogr. A 1218(43), 7886–7894 (2011).
(7) M. Ventura, B. Murphy and W. Goetzinger, J. Chromatogr. A 1220, 147–155 (2012).
(8) K. De Klerck, D. Mangelings, D. Clicq, F. De Boever and Y. Vander Heyden, J. Chromatogr. A 1234, 72–79 (2012).
(9) K.W. Phinney, L.C. Sander and S.A. Wise, Anal. Chem 70(11) 2331–2335 (1998).
(10) C.J. Welch, M. Biba, J.R. Gouker, G. Kath, P. Augustine and P. Hosek, Chirality 19(5), 184–189 (2007).
(11) C. Brunelli, Y. Zhao, M.H. Brown and P. Sandra, J. Chromatogr. A 1185(2), 263–272 (2008).
(12) W.W. Barnhart, K.H. Gahm, S. Thomas, S. Notari, D. Semin and J. Cheetham, J. Sep. Sci. 28(7), 619–626 (2005).
(13) T.A. Berger, Chromatographia 72, 597–602 (2010) .
(14) A. Grand-Guillaume Perrenoud, J.L. Veuthey and D. Guillarme, J. Chromatogr. A 1266, 158–167 (2012).
(15) E. Olah, S. Fekete, J. Fekete and K. Ganzler, J. Chromatogr. A 1217(23), 3642–3653 (2010).
(16) T.A. Berger, J. Chromatogr. A 1218(28), 4559–4568 (2011).
(17) S. Fanali, G. D'Orazio, T. Farkas and B. Chankvetadze, J. Chromatogr. A 1269, 136–142 (2012).
(18) K. Lomsadze, G. Jibuti, T. Farkas and B. Chankvetadze, J. Chromatogr. A 1234, 50–55 (2012).
(19) D. Mangelings and Y. Vander Heyden, J. Sep. Sci . 31(8), 1252–1273 (2008) .
(20) T.J. Ward and K.D. Ward, Anal. Chem 82(12), 4712–4722 (2010).
(21) L. Miller, J. Chromatogr. A 1256, 261–266 (2012).
(22) C.F. Poole, J. Chromatogr. A 1250, 157–171 (2012).
(23) C. West, S. Khater and E. Lesellier, J. Chromatogr. A 1250, 182–195 (2012).
(24) M.L. de la Puente, P. Lopez Soto-Yarritu and J. Burnett, J. Chromatogr. A 1218(47), 8551–8560 (2011).

Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.










