2D-LC–MS Approaches for the Analysis of In-Process Samples and for the Characterization of mAbs in a Regulated Environment
Biologics, and in particular monoclonal antibodies (mAbs), are an important class of therapeutics, and their market share keeps growing. The production of antibodies is a complex and lengthy process. In-process characterization of the mAb would help in optimizing the production steps. Efficiency in mAb characterization can be obtained by automating analysis and reducing hands-on time. Although mass spectrometry (MS) is an essential technique for detailed characterization of biomolecules, its use is limited to purified samples. However, the hyphenation of an MS system to two-dimensional liquid chromatography (2D-LC) allows for the analysis of more complex samples. The first dimension of a 2D-LC system can be used to purify the sample from its matrix or separate compounds using mobile phases that are not MS-compatible, whereas the second dimension coupled to MS can be used to desalt or separate the different variants or species obtained on the first dimension. A 2D-LC–MS system installed in a full good manufacturing practice (GMP)-compliant environment using validated software was used for the characterization of mAbs in complex mixtures at the intact and subunit levels using a Protein A affinity column with no sample preparation steps. In the second application, MS characterization of mAb subunits was made possible by digestion of the mAb online by an immobilized IdeS enzyme. The addition of a disulfide bridge reduction step online led to analyzing smaller molecules to access fine characterization.
Monoclonal antibodies (mAbs) represent a large part of pharmaceuticals currently under development (1). Monoclonal antibodies are 150 kDa molecules consisting of two heavy chains of ~50 kDa and two light chains of ~25 kDa, connected by disulfide bridges. The production of mAbs is a lengthy and complex process, taking place in several steps. However, it is important to follow the mAb critical quality attributes (CQA) along the production steps, and to assess that no modification or degradation has occurred on the protein. The presence of the mAb in a production medium is a hindrance to its rapid and efficient analysis. Techniques such as mass spectrometry (MS), commonly used to determine the molecular weight and the presence of modifications (2), require the use of purified samples, making sample preparation steps mandatory. These steps are timeconsuming, while fast characterization is expected. Online purification and sample preparation would be of great benefit to quickly characterize these products.
Typical modifications followed during the production process are the glycan composition, the presence of the terminal lysine on the heavy chains, the formation of pyroglutamic type of residues, or oxidation. These modifications can be determined by analyzing the molecule at the intact level, yet even more detailed information is obtained by working at the subunit level. Both chemical and enzymatic approaches are used to decrease the complexity of the analysis of the mass spectrometric data. Reduction of intramolecular disulfide bridges with a reducing agent, such as tris(2-carboxyethyl)phosphine hydrochloride (TCEP) or dithiothreitol (DTT), enables the analysis of light and heavy chains separately (25 and 50 kDa, respectively). This is commonly done offline in microtubes, but online reduction has already been reported by Camperi and associates (3).
Another way to obtain subunits is to enzymatically digest the mAb with, for instance, the IdeS enzyme (4), which cleaves above the hinge region. This leads to the formation of a subunit of ~100 kDa (Fab region) and two Fc/2 subunits of ~25 kDa. By using a reducing agent on this digested sample, three subunits can be obtained with average molecular weights of ~25 kDa: Fc/2, LC, and Fd.
Recent developments in chromatographic columns have made the purification and digestion of samples directly on a chromatographic system possible. For instance, pepsin columns have been largely used for many years for automated hydrogen deuterium exchange mass spectroscopy (HDX-MS) studies (5). There are also reports of trypsin columns used for online digestion of proteins (6). Such columns can be fitted on a twodimensional (2D) system, allowing for online sample preparation on the first dimension, followed by desalting or separation of the species on the second dimension (3). In other words, the flow at the output of the column used for purification or digestion purposes is coupled to a second column used for desalting or separation prior to MS analysis. A 2D liquid chromatography system (2DLC) coupled to a mass spectrometer enables the analysis of mAbs after online purification, using the second dimension of the system to desalt the sample or for further separation. This has been described previously with homemade systems adapted to the analysis (7).
Here, two applications are presented describing the use of a 2D-LC–MS system for MS characterization of mAbs without sample preparation in a fully good manufacturing practice (GMP)-compliant environment. The use of a high performance liquid chromatography (HPLC) column based on affinity allows for the purification of the mAb from its medium prior to MS analysis. Here, a Protein A affinity column was used in a two-dimensional LC system coupled to a mass spectrometer allowing for a fully automated analysis of the mAb without hands-on sample preparation. Furthermore, columns with immobilized enzymes are also a way to fasten analysis. For instance, a column with immobilized IdeS enzyme has been developed. The use of such a column on a 2D system with the addition of an online reduction step allows for the characterization of mAbs at the subunit level, with three species of ca. 25 kDa. In this range of molecular mass, excellent chromatographic resolution and mass accuracy can be obtained, allowing for detailed characterization of the mAb and of its quality attributes. These analyses, as well as data processing, are fully automated and can be performed using a fully GMP-compliant software and a qualified 2D-LC–MS system.
Materials and Methods
Materials
Adalimumab (Humira) and nivolumab (Opdivo) were used as model mAbs to perform these tests. All reagents and solvents were obtained from Sigma, Merck, or Biosolve. The 2D-LC–MS system used in this study consists of three pump systems (one quaternary pump [QSM] and two binary pump [BSM]), an autosampler, two Waters CM-A column managers (one used to connect the columns of each of the two dimensions and one used to fit seven trap columns with two valves: nine positions, eight ports), a photodiode array (PDA) detector, and a Xevo G2-XS QTof (Waters). The system was used in heart-cutting mode. Data were acquired and processed with the fully GMP-compliant UNIFI software (Waters). During development, samples were injected on the first dimension, and detected by UV to verify proper elution, equivalent to a first-dimensional (1D) system. In a 2D setup, samples are injected on the first-dimension column (1D), and by switching position on the valve of the column manager, the fractions of interest are sent to the trap columns. At the end of the 1 D gradient, the trap column can be flushed for desalting or used for online reduction for instance. Finally, the compounds are eluted from each trap column onto the second-dimension column (2D) by sending the flow through the output of the trap column towards the second-dimension column (2D). Detection was performed on the coupled ESI-QTOF-MS (Xevo G2-XS QTof) system. With this setup, up to seven trap columns can be connected, allowing a maximum of seven heart-cuts per sample injection.
Two-Dimensional Liquid Chromatography Coupled to Mass Spectrometry
Application 1: Characterization of mAbs Present in Complex Mixtures
Samples were injected “as is” (0.5 µg for analysis at the intact level, or 0.2 µg if reduction was performed) on the 2D-LC–MS system. On the first dimension, eluent A was phosphate buffer saline (PBS) at pH 7.4, and eluent B was PBS complemented with 12 mM hydrochloric acid (HCl) at pH 2.4. Elution on the MAbPac Protein A column (35 × 4 mm, 12-µm, Thermo Scientific) was in a step gradient with 1 min at 100% A, followed by 2.5 min at 100% B at 0.3 mL/min. Trapping was done with a BioResolve column (RP mAb polyphenyl, 30 × 2.1 mm, 2.7-µm, Waters). Online reduction of disulfide bonds was performed with a solution of 10 mM DTT in 50 mM ammonium bicarbonate set on line B with a flow of 0.5 mL/ min for 5 min, followed by a washing step of 10 min with 0.1% formic acid (FA) in H2O at 0.5 mL/min (line A). When on-trap reduction was not needed, desalting was performed for 10 min with 0.1% FA in H2O at 0.15 mL/min. For these steps, with or without reduction, the trap column was heated at 80 °C. On the second dimension, elution was performed on a BioResolve column (RP mAb polyphenyl, 50 × 2.1 mm, 2.7-µm, Waters) with 0.1% FA in H2O as eluent A, and 0.1% FA in acetonitrile as eluent B at 80 °C. Gradient for elution of intact mAbs was as follows: 2 min isocratic at 5% B; from 2 to 3 min, linear gradient to 20% B, followed by a linear gradient to 13 min to 90% B at 0.3 mL/min before washing the column and equilibration in the starting conditions. The gradient for elution of mAbs after reduction was as follows: 2 min isocratic at 5% B at 0.3 mL/min; from 2 to 3 min, linear gradient to 20% B at 0.2 mL/min followed by a linear gradient to 13 min to 40% B and linear gradient to 14 min to 90% B at 0.2 mL/min, before washing the columns and equilibration in the starting conditions. Total run time for such analysis was 45 min at the intact level, and 55 min at the reduced level.
Mass spectra were acquired on an online Xevo G2-XS (Waters) coupled to the 2D-LC–MS system, equipped with a z-spray electrospray ionization source, a quadrupole, and an orthogonal time of flight. For the analysis of mAbs at the intact level, capillary voltage was set at 2.75 kV, cone voltage at 150 V, source temperature at 100 °C, desolvation temperature at 600 °C, cone gas flow at 0, and desolvation gas flow at 800 L/h. For the analysis of mAbs subunits, capillary voltage was set at 2.5 kV, cone voltage at 120 V, source temperature at 100 °C, desolvation temperature at 500 °C, cone gas flow at 100, and desolvation gas flow at 1000 L/h.
Application 2: Automated Characterization of mAbs at the Subunit Level:
Samples (1 µg) were injected onto the system. On the first dimension, eluent A was 200 mM ammonium acetate. Isocratic elution was performed on a HPLC Fabricator column (Genovis) at 37 °C for 20 min. On the BSM used for fitting the trap columns, a Bioresolve RP mAb polyphenyl (30 × 2.1 mm, 2.7-µm, Waters) was installed and 0.05% FA + 0.05% trifluoroacetic acid (TFA) in H2O was set on line A. Online reduction of disulfide bonds was performed with a solution of 10 mM DTT in 50 mM ammonium bicarbonate set on line B, with a flow of 0.5 mL/min for 5 min, followed by a washing step of 10 min with 0.05% FA + 0.05% TFA in H2O at 0.5 mL/min. If no reduction was needed, no washing was done before elution on the second dimension. The trap column was heated at 80 °C. On the second dimension, elution was performed on a MabPac RP (50 × 3 mm, 4-µm, Thermo Scientific) column with 0.05% FA + 0.05% TFA in H2O as eluent A, and 0.05% FA + 0.05% TFA in acetonitrile as eluent B. The gradient was as follows: isocratic for 2 min at 5% B at 0.3 min, linear gradient from 2 to 3 min at 0.2 mL/min to 28% B, linear gradient to 23 min to 36% B at 0.2 mL/min. Elution was performed at 80 °C. Mass spectra were acquired on the Xevo G2-XS coupled online to the 2D-LC system with the same parameters as described above for subunits.
To induce oxidation, nivolumab was incubated with H2O2 for 45 min at RT in the presence of 0, 0.075, or 0.2% H2O2 . The oxidation reaction was stopped by addition of methionine to the solution. Samples were injected “as is” to the 2D-LC–MS system without further preparation for online IdeS digestion with and without DTT reduction and with the optimal conditions defined above.
Results and Discussion
Implementing Online Reduction
Tests were performed to evaluate the feasibility of online reduction of disulfide bridges present on mAbs on the 2D-LC system. The reduction reaction was performed after the heart-cut, when the fraction of interest is on the trap column, prior to elution on a second dimension. Initial disulfide bridge reduction tests were performed with TCEP because it is known to be a good reducing agent, independently of the pH of the solution. Tests were performed by injecting the mAb directly on the trap column without using a column on the first dimension for development method purposes. The initial runs consisted of a flow of 0.5 mL/min of 1 mM TCEP in 10:90 (v/v) acetonitrile–water sent through the trap column for 5 min while the compound was trapped. At this stage, three trap column temperatures (room temperature, 50 °C, and 80 °C) were tested. Both the light and heavy chains were present, indicating that the reduction was successful. However, for each of the subunits, multiple peaks were observed. Based on the analysis of the mass spectra, it was found that intramolecular disulfide bridges were not completely reduced. This was not improved by increasing the flushing time of TCEP to 20 min or the concentration of TCEP to 20 mM. This phenomenon has been previously described in the literature (3).
As a result, further tests were performed with DTT. One disadvantage of this reducing agent is that its optimum activity is at pH 8 and its stability is limited (8). This means that a buffer needs to be used to control the pH. DTT was prepared in 50 mM ammonium bicarbonate and, as described above, a flow of 10 mM DTT in ammonium bicarbonate was sent to the trap column for 5 min. In these conditions, only one peak for each light and heavy chain was observed (Figure 1). Complete reduction was confirmed by deconvoluting the MS spectra obtained, in which only the species with all disulfide bridges reduced were found. Furthermore, after keeping the DTT solution on the system overnight at room temperature, comparable efficiency in the reduction of the mAb was obtained. This indicates that such solutions can be used for a long sequence in which multiple samples are analyzed. This successful online reduction was further used as a step in a full 2D-LC–MS analysis.
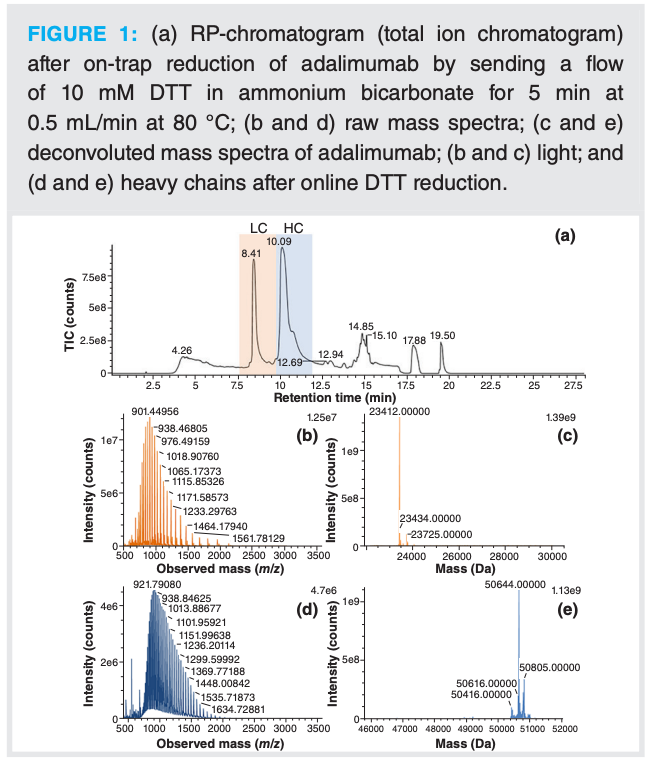
Characterization of mAbs in Complex Mixtures
In the development phase, elution on the Protein A column was tested in a 1D setup using UV detection to ensure proper elution of the mAb from the Protein A column. Good reproducibility (0.2% RSD on UV signal for triplicate injection) was observed, as well as linearity of the UV signal as a function of the injected amount. After this successful step, the complete 2D setup was used by injecting adalimumab. As proper elution and MS signal were obtained for the mAb, the influence of the starting medium was tested. The elution of adalimumab diluted in water and adalimumab diluted in a complex cell culture medium were compared. There was no influence of the starting solution on the elution on the second dimension and on the MS data. The same retention time, peak shape, and deconvoluted masses were observed.
It was found during the different tests performed that the desalting step (flushing of the trap column while the mAb was trapped) had an impact on peak tailing in the second dimension. Extensive desalting with high flow rate or duration led to a large tailing of the mAb peak. To avoid such peak tailing, the desalting step was initially removed. By looking closely at the spectra, it became clear that desalting was necessary to obtain a mass spectrum of acceptable quality that could be correctly deconvoluted. Several adjustments were needed to have both limited tailing and good MS data quality. The influence of the duration and flow rate of the desalting step was evaluated. Eventually, a desalting step of 10 min at a moderate flow rate of 0.15 mL/min was found to be a good compromise in addition to optimized MS parameters (Figure 2).
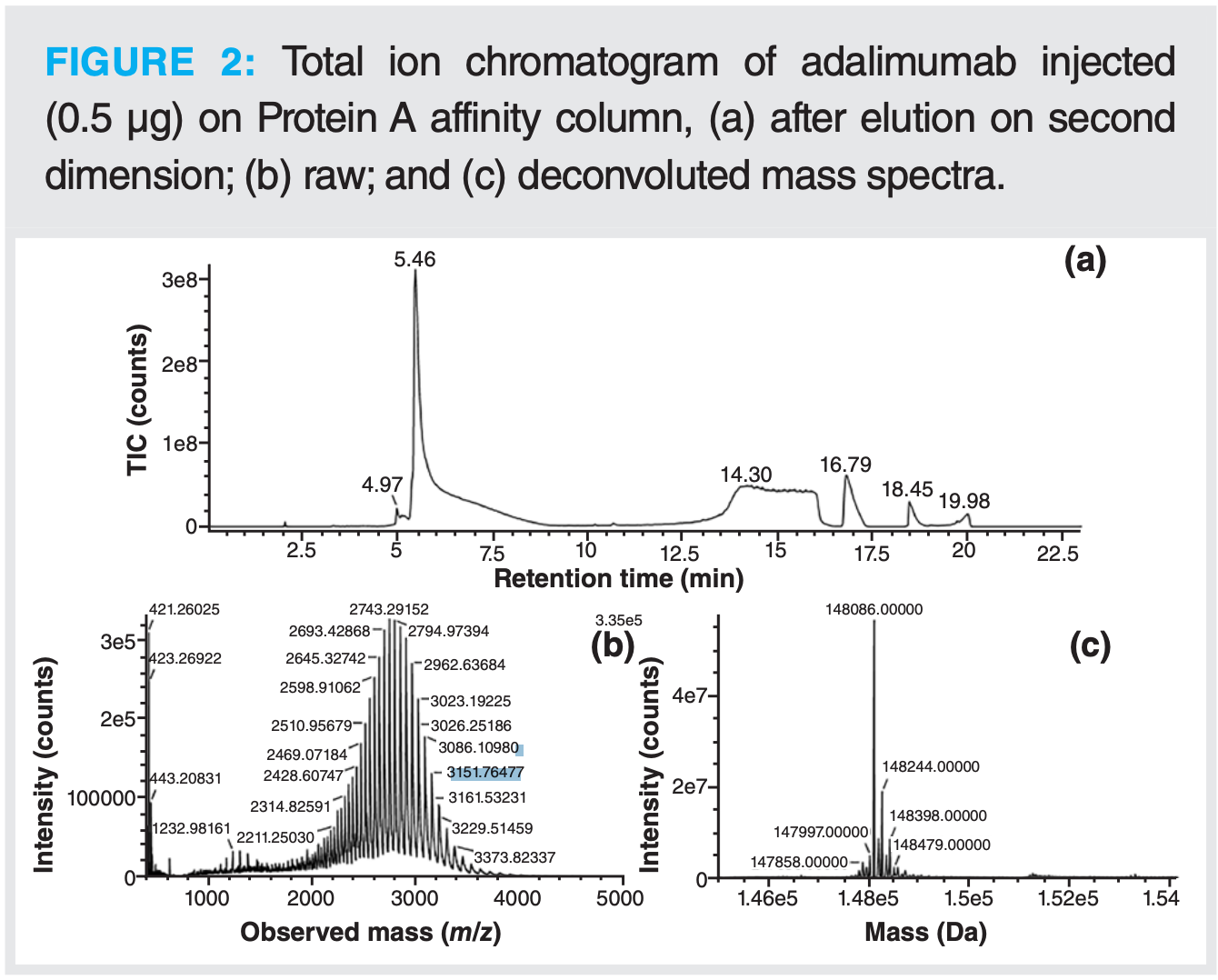
After this, the online reduction step with DTT, as described above, was tested in a full 2D-LC setup. As observed for the test without a column on the first dimension, the light and heavy chains were well separated, and all disulfide bridges (inter- and intramolecular) were fully reduced (Figure 3). For this type of analysis, even less material is needed than for intact analysis. It was indeed observed that injected amounts higher than 0.4 µg led to saturation of the MS signal.
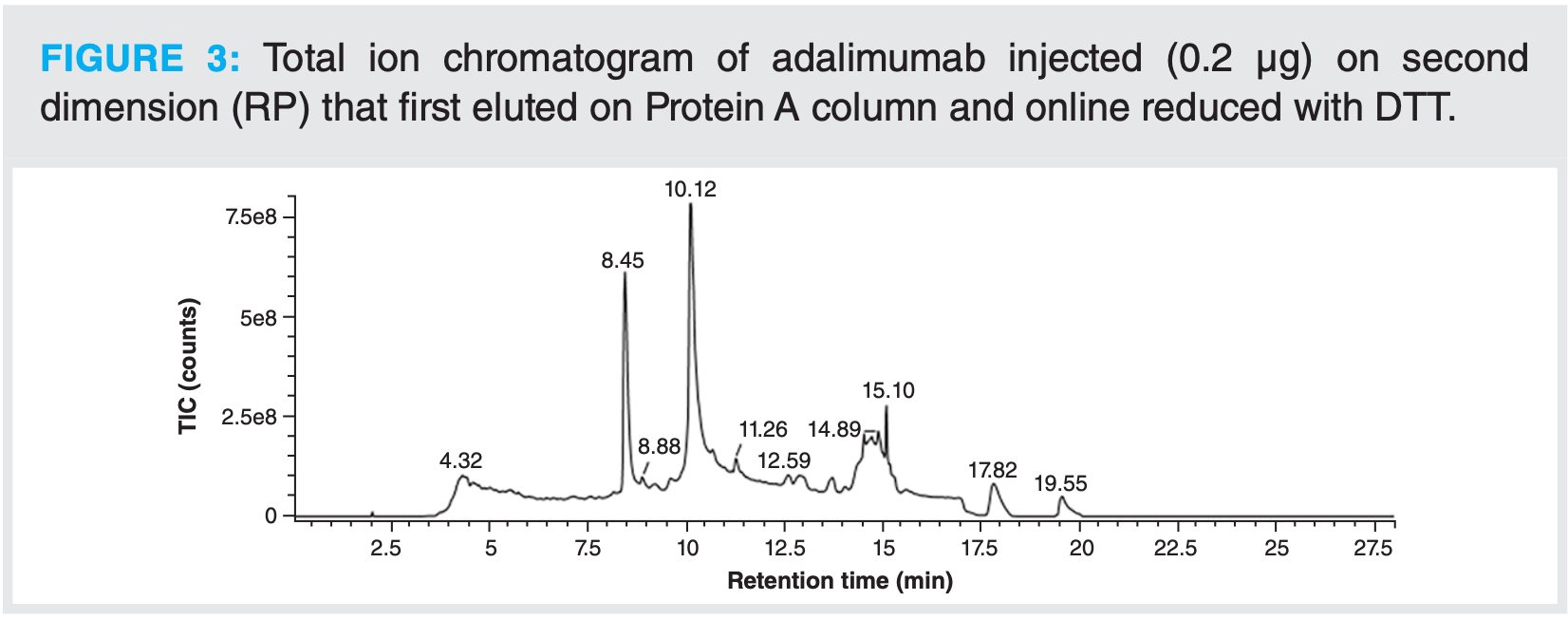
In conclusion, a fast method was developed for the analysis of mAbs at the intact or subunit levels present in complex mixtures. Peak identification was performed automatically with UNIFI software. Within less than 1 h, without prior sample preparation, using a fully automated and GMP-compliant system, MS data were obtained, thereby allowing for a full characterization of the antibody.
Automated MS Characterization of mAbs at Subunit Level
Development
The analysis of mAbs at the subunit level was performed using an immobilized IdeS column. Elution on this column is isocratic and digestion occurs using a low flow, so there is enough time for proper digestion. Initial tests only consisted in checking that the mAb would elute properly from the column using the 1D setup with UV detection. For these first steps, a mobile phase consisting of PBS was used and adalimumab was chosen as model mAb. By working with the complete 2D-LC–MS system, it was confirmed that the IdeS digestion was complete as the expected F(ab’)2 and Fc/2 species were observed. Extrapolation was performed by working with another mAb, namely nivolumab, and by including the online DTT reduction step as described above. No intact nivolumab was observed after IdeS digestion only, but the presence of heavy chain was detected after enzymatic digestion followed by reduction. The presence of the heavy chain shows that the enzymatic digestion was incomplete, as the IdeS enzyme is cleaving above the hinge region (Figure 4).
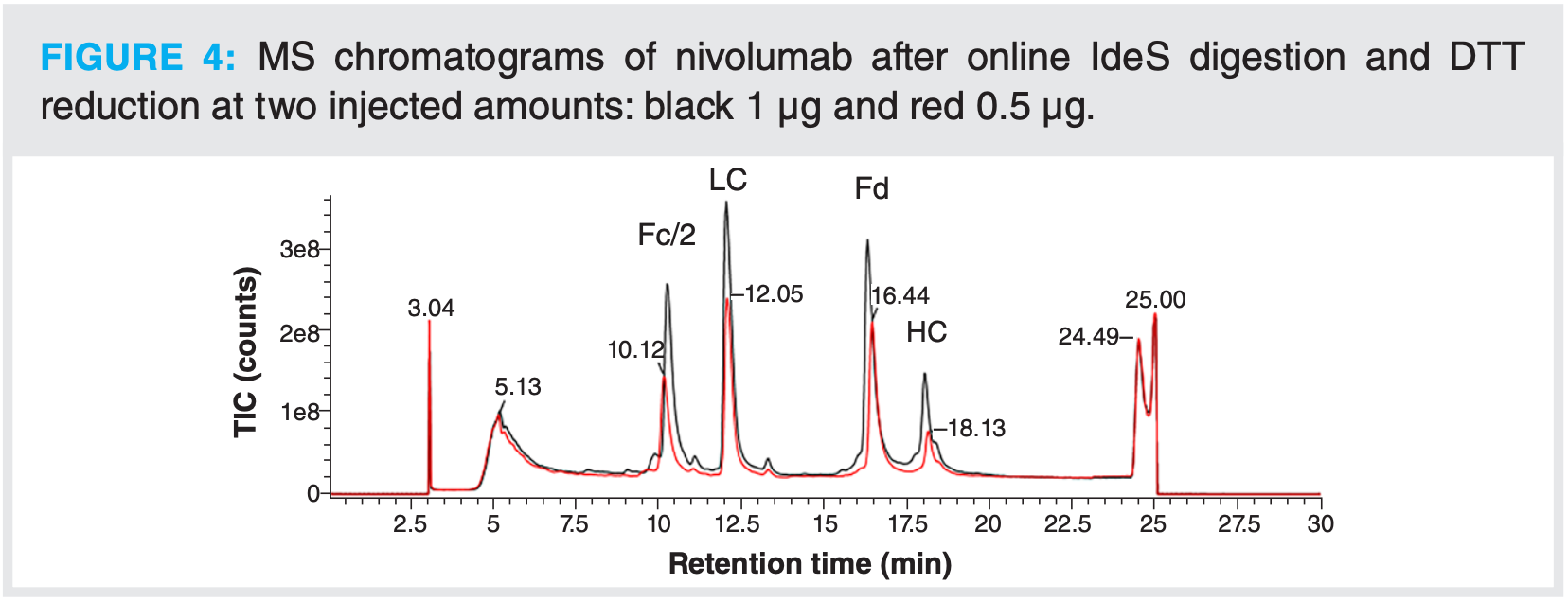
To try and solve this issue, several tests were performed such as lowering the flow rate on the Fabricator column or by injecting lower amounts of nivolumab. Still, the presence of heavy chain was detected. It has been described that one way to improve the digestion is to increase the ionic strength (9), but the digestion was still incomplete using PBS 2×. At this stage, it was decided to test a different eluent. By using ammonium acetate, digestion was complete, but concentration had to be increased to 200 mM (from 150 mM tested initially) to obtain a sharp peak in the first dimension, which would allow for a clean heart-cut.
Case Study: Quantifying Oxidation on Nivolumab
Oxidation was induced at three levels to evaluate the potential of such an automated method for detection and quantification of these type of modifications. Oxidation mainly occurs on the Fc part of the antibody. The oxidized forms elute earlier than the non-modified Fc/2 fragments (Figure 5). It is even possible on this example to distinguish between mono-oxidized and di-oxidized species. The different compounds were identified automatically with UNIFI software. Based on these identifications, the oxidation ratio was calculated. In this example, with rather high H2O2 concentrations, oxidation up to 74% was calculated on the Fc/2 species after IdeS digestion and online reduction (Figure 6). For the samples that were only digested with IdeS, the same trend of oxidation level as a function of H2O2 concentration was observed. Calculated levels were, however, slightly higher, with an oxidation of 86% calculated for the highest level of H2O2.
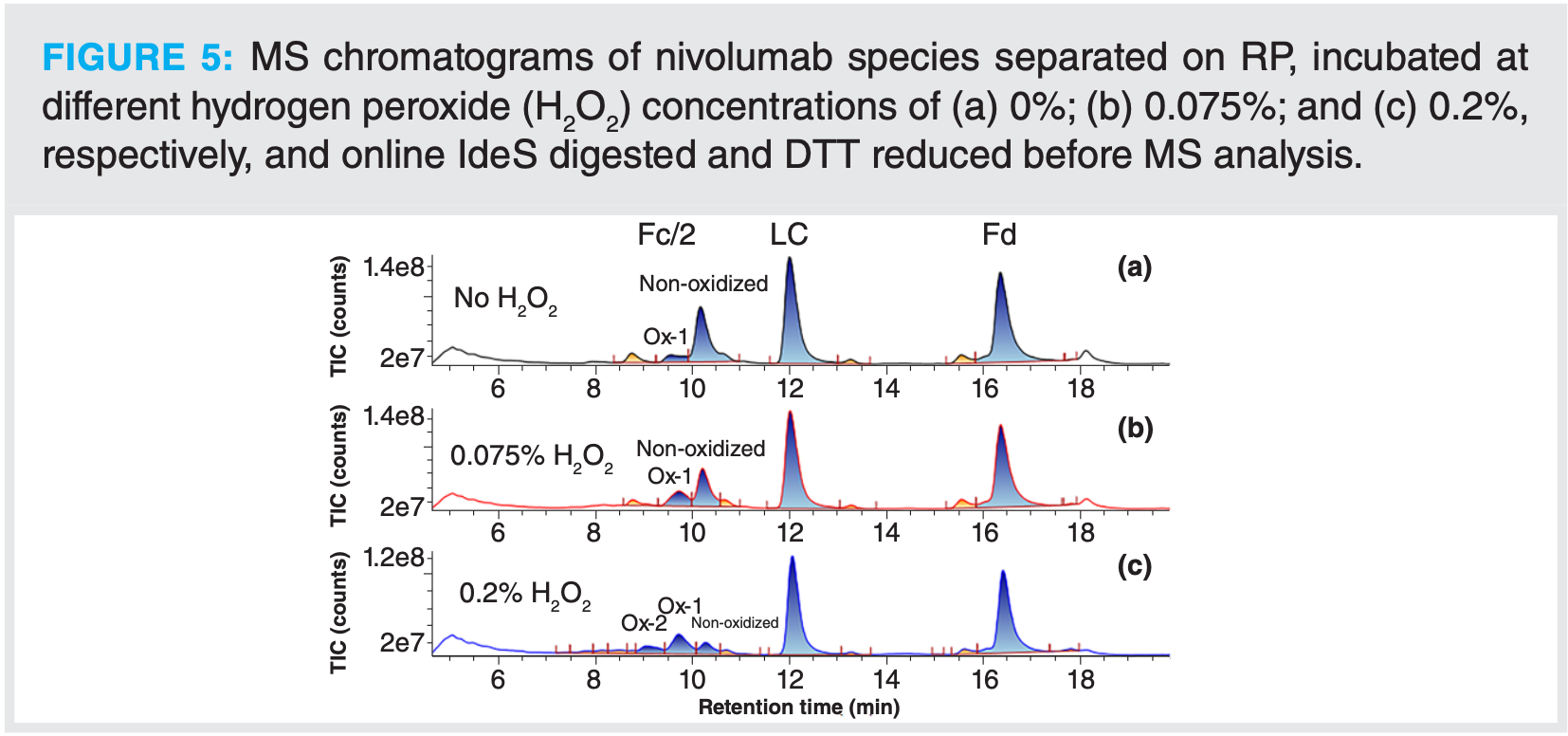
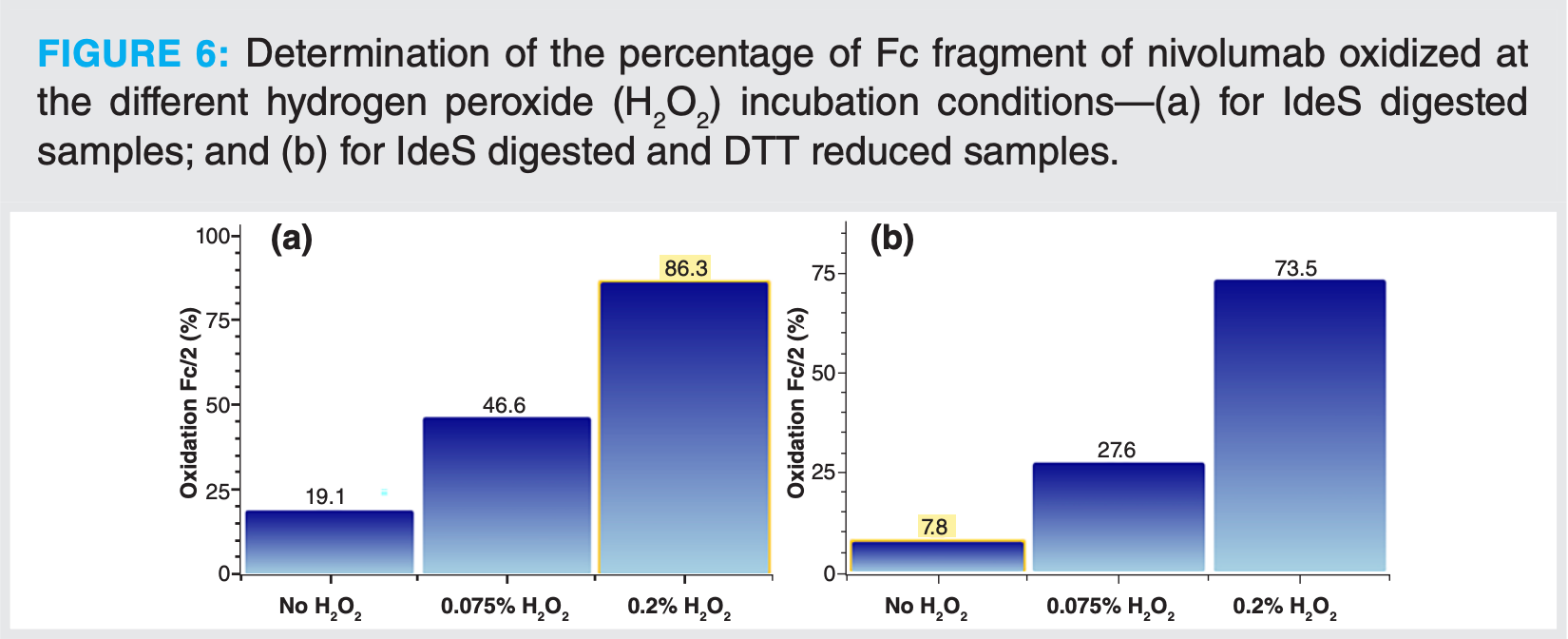
With this example, it was demonstrated that without prior sample preparation, modifications present on mAbs can be easily determined. The level of modification was quantified by mass spectrometry at the subunit level using a 2D-LC–MS workflow.
Conclusion
Two approaches were developed to characterize mAbs with mass spectrometry using innovative two-dimensional liquid chromatography workflows. For both approaches, detailed information on monoclonal antibodies can be obtained at the intact or subunit levels without any sample preparation, using a fully automated and GMP-compliant 2D-LC–MS system.
With these techniques, optimization of the mAb production process is made easier and faster by analyzing samples without manual purification. The second approach shows the potential for automated quality control (QC) multiple quality attributes monitoring of mAbs without any sample preparation.
These methodologies open the way to fully compliant at-line monitoring of monoclonal antibody quality attributes.
References
(1) Castelli, M. S.; McGonigle, P.; Hornby, P. J. The Pharmacology and Therapeutic Applications of Monoclonal Antibodies. Pharmacol. Res. Perspect. 2019, 7 (6), e00535. DOI: 10.1002/prp2.535
(2) Zhang, Z.; Pan, H.; Chen, X. Mass Spectrometry for Structural Characterization of Therapeutic Antibodies. Mass Spectrom. Rev. 2009, 28, 147–176. DOI: 10.1002/mas.20190
(3) Camperi, J.; Dai, L.; Guillarme, D.; Stella, C. Development of a 3D-LC/MS Workflow for Fast, Automated, and Effective Characterization of Glycosylation Patterns of Biotherapeutic Products. Anal. Chem. 2020, 92, 4357–4363. DOI: 10.1021/acs.analchem.9b05193
(4) von Pawel-Rammingen, U.; Johansson, B. P.; Björck, L. IdeS, A Novel Streptococcal Cysteine Proteinase with Unique Specificity for Immunoglobulin G. EMBO J. 2002, 21, 1607–1615. DOI: 10.1093/emboj/21.7.1607
(5) Narang, D.; Lento, C.; Wilson, D. J. HDX-MS: An Analytical Tool to Capture Protein Motion in Action. Biomedicines 2020, 8, 224. DOI: 10.3390/BIOMEDICINES8070224
(6) Šlechtová, T.; Gilar, M.; Kalíková, K.; et al. Performance Comparison of Three Trypsin Columns Used in Liquid Chromatography. J. Chromatogr. A 2017, 1490, 126–132. DOI: 10.1016/j.chroma.2017.02.024
(7) Camperi, J.; Dai, L.; Guillarme, D.; Stella, C. Fast and Automated Characterization of Monoclonal Antibody Minor Variants from Cell Cultures by Combined Protein-A and Multidimensional LC/MS Methodologies. Anal. Chem. 2020, 92, 8506–8513. DOI: 10.1021/acs.analchem.0c01250
(8) Getz, E. B.; Xiao, M.; Chakrabarty, T.; Cooke, R.; Selvin, P. R. A Comparison between the Sulfhydryl Reductants Tris(2-carboxyethyl) phosphine and Dithiothreitol for Use in Protein Biochemistry. Anal. Biochem. 1999, 273, 73–80. DOI: 10.1006/abio.1999.4203
(9) Nägeli, A.; Björk, S.; Nordgren, M.; Nyhlén, H. Automated Middle-level Antibody Analysis Using On-Column FabRICATOR Digestion with LC-MS. Genovis AB Application Note. https://www.genovis.com/wp-content/uploads/genovis-application-note-fabricatorhplc-06.pdf (accessed 2023-09-15).
About the Authors
Claire I. Butré and Arnaud Delobel are with Quality Assistance in Donstiennes, Belgium.

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.












