Utility of UPLC–MS–MS and SPE for High Throughput Quantitative Bioanalysis
The Application Notebook
The use of 30 mm UPLC columns coupled with Oasis SPE in µElution format was investigated to increase the speed of quantitative bioanalytical methods while maintaining sensitivity and resolution of closely related analytes.
Introduction
Bioanalytical laboratories are required to develop fast LC methods which still separate analytes, closely related compounds and metabolites from endogenous components. Many of the challenges of developing a bioanalytical method revolve around meeting the rigorous criteria set forth in the FDA Guidance for Industry for Bioanalytical Method Validation, including the investigation of matrix effects. Both good chromatography and sample preparation are necessary for ensuring rugged bioanalytical methods. Two representative mixtures of closely related analytes were chosen. Mixture 1 contains imipramine, D4-imipramine, and clomipramine. Mixture 2 contains risperidone, 9-OH risperidone (the major circulating metabolite), and clozapine. This work demonstrates the utility of sub-2 μm chromatographic particles packed in short columns coupled to highly selective mixed-mode sample preparation for the development of quantitative bioanalytical methods.
Experimental Conditions
Extraction Procedure
Oasis MCX μElution 96-well plate (2 mg/well of sorbent):
- Condition with 200 μL MeOH.
- Equilibrate with 200 μL water.
- Load 400 μL diluted plasma sample (200 μL plasma + 200 μL 4% phosphoric acid).
- Wash with 200 μL 2% HCOOH in water.
- Wash with 200 μL MeOH.
- Elute with 2 × 25 μL 5% NH4OH in 90:10 MeOH:H2O for the risperidone mixture or 2 × 25 μL 5% NH4OH in ACN for the imipramine mixture.
- Dilute with 50 μL water.
Results and Discussion
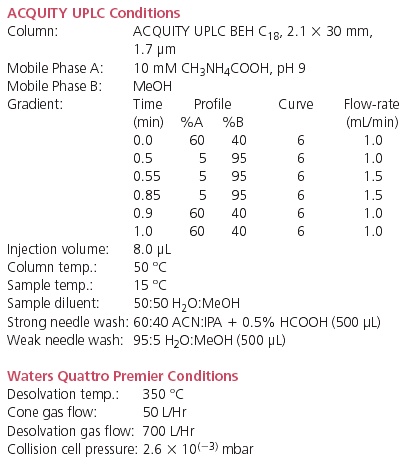
To selectively separate analytes from matrix components, mixed-mode SPE was used because of its retention by both reversed-phase and ion-exchange mechanisms. SPE recoveries for all six analytes were >90% for extracted human plasma samples. A representative chromatogram of imipramine, the closely related compound clomipramine, and the internal standard D4-imipramine at 0.1 ng/mL in extracted human plasma is shown in Figure 1. A representative chromatogram of risperidone, its hydroxylated metabolite, and the internal standard clozapine at 0.1 ng/mL in extracted human plasma is shown in Figure 2. Sample preparation was demonstrated to be selective for the analytes of interest, as average matrix effects were <15% for the six compounds evaluated, even under these rapid gradient conditions.
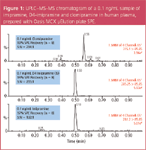
Figure 1
To obtain a cleaner final extract for analysis of the risperidone mixture, the elution solvent was modified from 5% NH4OH in 100% MeOH to 5% NH4OH in 90:10 MeOH:H2O. Using less organic solvent in the final eluate more selectively elutes the analytes while retaining very hydrophobic interferences on the SPE sorbent. For the imipramine mixture, the elution solvent was modified from 5% NH4OH in 100% MeOH to 5% NH4OH in 100% ACN. Imipramine and the related compounds are very hydrophobic and require a strong elution solvent to fully desorb from the SPE sorbent. Previous work has shown that plasma phospholipids,1 some of the compounds most heavily implicated in matrix effects, are more soluble in protic solvents such as methanol and less soluble in aprotic solvents such as acetonitrile. For this reason, acetonitrile was substituted for methanol in the final elution, resulting in a cleaner final extract for LC–MS–MS analysis. SPE was performed on a 96-well μElution plate format which enables elution in volumes as low as 10–25 μL. This provides sample concentration without drying down and reconstituting the extract, resulting in improved sample throughput and increased sensitivity.
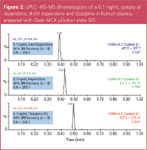
Figure 2
Chromatography was performed on a 30 mm UPLC column to reduce cycle time. A total cycle time of only 1 minute adequately separated analytes from eachother and any endogenous interferences that may have been present.
Conclusions
The combination of UPLC technology, short columns, and Oasis SPE in μElution plate format offers significant advantages to bioanalytical scientists. The sub-2 μm particles used in UPLC allow for increased resolution at higher linear velocities, which enables method development scientists to shorten run times without sacrificing separations of closely related analytes. The added resolution of UPLC also allows the use of 30 mm columns instead of 50 or 100 mm to further shorten LC run times. As demonstrated in this work, closely related analytes were separated in under a minute with this combination. Such short LC run times increase the need for effective sample preparation. The use of mixed-mode Oasis SPE in μElution plate format achieves both sample pre-concentration and cleaner extracts, which minimizes matrix effects, even under very rapid gradient conditions. The combination of selective sample preparation and the speed and sensitivity of the UPLC/Quattro Premier platform facilitate rapid development of robust bioanalytical methods.
Erin E. Chambers and Diane M. Diehl, Waters Corp., Milford, Massachusetts, USA.
References
1. E. Chambers, D. Wagrowski-Diehl, Z. Lu and J. Mazzeo, J. Chromatogr. B, 852(1–2), 22–34 (2007).
© 2007 Waters Corporation. Waters, The Science of What's Possible, UPLC, Oasis, ACQUITY UPLC and Quattro Premier are trademarks of Waters Corporation.

Waters Corporation
34 Maple Street, Milford, Massachusetts 01757, USA
tel. +1 508 478 2000 fax +1 508 478 1990
Website: www.waters.com

The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.
MilliporeSigma: Ultrapure Water for Sensitive LC-MS Analysis of Pesticides
March 25th 2025The aim of the study was to illustrate the efficiency of Milli-Q® water purification systems in eliminating pesticides from tap water, thereby producing and delivering reliable and consistent-quality ultrapure water suitable for pesticides analysis








