Analysis of Biodiesels Using LC–MS
The Application Notebook
Preliminary studies of biodiesel samples by a high speed LC–MS system using electrospray ionization and a patented cone-wash feature demonstrate that LC–MS reduces the analysis time to 20 minutes and reveals information about higher molecular weight compounds in biodiesel while still detecting many low molecular weight chemicals, including FAMEs, at high sensitivity.
Experimental Conditions
The method was developed using a Thermo Scientific Accela high speed LC and the MSQ Plus single quadrupole. This system had a pressure tolerance of 15000 psi enabling flexible control of the chromatographic parameters.
The integrated cone wash delivered a continuous 80 μL/min of methanol to the skimmer cone, eliminating any possible deposition and blockage at the entrance cone. Direct infusion of samples and standards was used for instrument tuning and optimization of the MS parameters.
The biodiesel sample was mixed in winter diesel at 5% (BD5). They were diluted in methanol to 100 mg/mL and further diluted to 1 mg/mL in 10 mM LiI in methanol. Tricaprin and monoolein were diluted in methanol to 10 mg/mL solutions. They were further diluted in methanol in series to the final concentration of 10 mM LiI and 0.005, 0.010, 0.050, 0.1, 2.5 and 5 mg/mL of tricaprin and 10 mM of LiI and 0.001, 0.005, 0.01, 0.05, 0.1, 0.5, 1, 2.5 and 5 mg/mL of monoolein.
Results
A clean separation of the sample was obtained within 20 minutes [see Figure 1(a)]. The mass spectrum in Figure 1(b) illustrates that there were many low molecular weight (MW) components, as well as many high MW components. Essentially, the low MW components came out in the first part of the chromatogram (there are more than 20 different identifiable compounds before 13 min), while the higher MW components came out in the second part (>14 min). In this separation, the MS was more sensitive to this type of biodiesel sample than a PDA detector. While we saw more than 15 peaks in the TIC acquired by the MSQ Plus, there were only three identifiable UV peaks in the 205 nm trace. They represented the major FAMEs components in biodiesel.
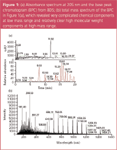
Figure 1
The above results cannot be achieved without the cone-wash feature (exclusively available on the MSQ Plus) because the cone wash removes any possible residue deposition to the entrance cone of the MSQ Plus, keeping it from clogging.
Conclusions
Preliminary studies of biodiesel samples by a high speed LC–MS system demonstrate that LC–MS can reduce the analysis time to 20 minutes and also reveals information about higher MW compounds in biodiesel while still detecting many low MW chemicals, including FAMEs, at high sensitivity. The patented cone-wash integrated into the electrospray source of the mass spectrometer allowed for the uninterrupted analysis of complicated sample matrices and kept the interface clean from contamination. The methodology described in this publication can be used to characterize chemicals in a wide range of petroleum and petrochemical analyses.
Jennifer Huang, Ray Chen, Daniela Cavagnino and Ed Long, Thermo Fisher Scientific, San Jose, California, USA.

Thermo Fisher Scientific
355 River Oaks Parkway, San Jose, California 95134, USA
tel. +1 561 688 8700, fax +1 608 273 6880
E-mail: analyze@thermofisher.com
Website: www.thermo.com/lc

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)






