Improved LC–MS Performance — A Three-Step Method for Minimizing Background Signals and Increasing Ionization Efficiency
The Application Notebook
One problem frequently encountered in LC–MS is the appearance of mass peaks, which appear totally unrelated to the samples run - "ghost" mass peaks. It is impossible to differentiate whether these signals come from an unknown component in the sample co-eluting with a known peak, or from an impurity in the mobile phase or from some residual contamination "bleeding" from the column.
One problem frequently encountered in LC–MS is the appearance of mass peaks, which appear totally unrelated to the samples run — "ghost" mass peaks. It is impossible to differentiate whether these signals come from an unknown component in the sample co-eluting with a known peak, or from an impurity in the mobile phase or from some residual contamination "bleeding" from the column.
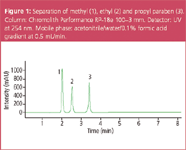
Figure 1
To solve this problem, a three-step procedure is proposed for washing columns and choosing solvents for use in LC–MS measurements. This procedure also increases ionization efficiency and thereby increases both sensitivity and reproducibility of LC–MS measurements.
Step 1: Procedure for "Washing" the HPLC Column
A clean HPLC column is an essential pre-requisite for good LC–MS results. However, trace residues of organic compounds are nearly always present on reversed-phase HPLC columns. Residues may come from previously injected samples, from solvent impurities or from the silanes bonded to the silica surface. These residues are typically eluted very slowly from the column by the mobile phase. This phenomenon is called "column bleeding" and can give rise to unwanted background detector signals and can be very disturbing in LC–MS.
To minimize or even eliminate this "column bleeding", a simple procedure is to pump iso-propanol with 0.1% formic acid at room temperature for one hour through the column. For a column with 3 mm i.d., 0.5 mL/min flow-rate is appropriate. It is important that the column is not connected to the mass spectrometer to avoid contaminating the ion source.
Step 2: The Choice of Solvent Grade for the Mobile Phase
HPLC gradient or isocratic grade solvents are generally specified for use with UV detectors, sometimes with fluorescence detectors, but are not specified for use in LC–MS. Trace organic impurities may not absorb in UV at the wavelength used, but may be ionized in the mass spectrometer, thereby giving rise to background signals which appear in the mass spectra, making the spectra complex and difficult to interpret. Such impurities "compete" with the sample components in the ionization process and this competition causes "ionization suppression". The ionization efficiency is reduced and sensitivity is lost. Figure 2 shows a typical mass spectrum with low signal-to-noise ratio using standard reagent grade acetonitrile.
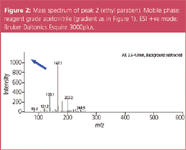
Figure 2
Figure 3 shows the same spectrum measured using LC–MS grade acetonitrile (Merck LiChrosolv hypergrade 1.00029). The ionization efficiency is increased more than 10-fold. This is a critical performance improvement. In addition, the intensity of background signals is negligible and all mass signals* can be directly correlated to the target compound, ethyl paraben.
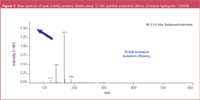
Figure 3
*(mass 167.1 = M+H, mass 189.0 = M+Na, mass 139.1 = fragment protonated p-hydroxybenzoic acid, mass 121.2 = protonated fragment)
Step 3: Equilibrating the HPLC Column
Finally the column must be re-equilibrated with the mobile phase. Best results are obtained when two blind gradients (without sample injection) are run prior to analysing samples.
Conclusion
By application of a simple column washing procedure and use of LC–MS specified solvent, LC–MS results can be improved in three ways:
- improved ionization efficiency and sensitivity
- improved reproducibility
- removal of unwanted background mass signals
The method has been verified with Chromolith RP-18e (monolithic silica) and Purospher STAR RP-18e 3 μm and 5 μm HPLC columns.
Footnotes
1. Solvents available from Merck in LC–MS specified quality: Acetonitrile — LiChrosolv hypergrade 1.00029 Methanol — LiChrosolv hypergrade 1.06035
2. Chromolith RP-18e columns are made from high-purity metal-free monolithic silica and used for fast LC–MS Purospher STAR RP-18e columns are made from high-purity metal-free 3 μm and 5 μm silica particles
Alexander Kraus, Rod McIlwrick, Gisela Jung and Ines Hädrich, Merck KGaA, Darmstadt, Germany.

Merck KGaA
Frankfurter Strasse 250, D-64293 Darmstadt, Germany
Fax: +49 6151 72 6080
E-mail: chromatography@merck.de
Websites: www.merck.de, www.chromatography.merck.de.

The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.
MilliporeSigma: Ultrapure Water for Sensitive LC-MS Analysis of Pesticides
March 25th 2025The aim of the study was to illustrate the efficiency of Milli-Q® water purification systems in eliminating pesticides from tap water, thereby producing and delivering reliable and consistent-quality ultrapure water suitable for pesticides analysis







