The Unique Selectivity of π-Interactions for Solid-Phase Extraction
LCGC Europe
Hypercrosslinked polystyrene-type (solid-phase extraction) SPE materials exhibit a unique ability to enter p-interactions with aromatic, heterocyclic and unsaturated compounds. This property permits selective extraction and pre-concentration of the above classes of species from non-polar media and fatty matrices. The principle has been exploited for developing analytical protocols to determine polar furan derivatives in mineral transformer oil, polyaromatic hydrocarbons (PAHs) in smoked fish and for the fractionation of polychlorinated aromatic compounds in environmental matrices.
Hypercrosslinked polystyrene-type (solid-phase extraction) SPE materials exhibit a unique ability to enter π-interactions with aromatic, heterocyclic and unsaturated compounds. This property permits selective extraction and pre-concentration of the above classes of species from non-polar media and fatty matrices. The principle has been exploited for developing analytical protocols to determine polar furan derivatives in mineral transformer oil, polyaromatic hydrocarbons (PAHs) in smoked fish and for the fractionation of polychlorinated aromatic compounds in environmental matrices.
Although remarkable progress has been made in solid-phase extraction (SPE), many applications still cause a lot of problems for the analyst. Non-polar matrices, such as edible and industrial oils, fats and petroleum, as well as methods that involve hexane extraction (for example, soil, food, plants and blood analysis) are particularly problematic. This often makes the analysis of non-polar analytes in non-polar matrices difficult. The problem can be acute in the following conditions:
i) If the presence of residual amounts of matrix compounds — such as long-chain hydrocarbons, triglycerides and other lipids — in the final sample cannot be tolerated by subsequent analysis methods.
ii) When both analytes and matrix components have low and similar polarities.
iii) If the concentration of analytes in the sample extract is very low and it is necessary to increase it in the final analytical sample.
iv) If preliminary fractionating of sample extract is necessary to obtain some separate fractions with different groups of analytes.
v) When the analytical procedure must meet some special rigid requirement, for example, to save time and cut labour costs, to be inexpensive, very simple, "green" etc.
It can be an extremely exacting task to develop a good analytical method combining all the points above. For example, let's consider the problem of solving i, ii and iii. Together i and ii mean that liquid–liquid extraction (LLE) is an unsuitable technique because the extract would be contaminated by matrix components. But is this also the case for solid-phase extraction? There are currently plenty of adsorption materials for SPE and the most commonly used adsorbents belong to one of three main types:
- Polar inorganic (silica, florisil, silica-based NH2, CN,)
- Strong cation exchange (SCX) and strong anion exchange (SAX)
- Non-polar composites (silica-based C1–C18); and organic polymeric materials.
Polymers with a low to modest degree of crosslinking swell in many non-polar solvents and then cease functioning as adsorbents and alkyl-silica (C1–C18) does not adsorb non-polar compounds from non-polar solvents. As a rule, polar materials can be used to remove polar analytes from hexane extracts, but they cannot be employed if the analytes are non-polar. Hence, most of the adsorbents mentioned previously for SPE are unsuitable for pre-concentration of contaminants in these circumstances,
Fortunately, in some instances, it is possible to overcome the problems highlighted in i, ii and iii. If the analytes and contaminating compounds with similar polarities have different aromatic (or unsaturated) moieties it may be possible to separate them from each other using a separation principle based on π-interactions. Such separations can be performed using available SPE adsorbents such as hypercrosslinked polystyrenes and graphitized carbon.
π-Interactions
Uncharged molecules can experience attractive interactions involving the outer electronic shells of their constituting atoms or atomic groups. In general, every pair of interacting molecules can be regarded as a complex of a Lewis base (donor of electrons) and Lewis acid (acceptor of electrons). This type of molecular interaction is called "charge-transfer" (CT) interaction as it involves the partial shift of electron density from one molecule to another. Two molecules can also form a complex using electrostatic interactions if they have permanent or induced dipoles or electric charges (in case of molecular ions). This article will focus on applying CT interactions to enhance selectivity in SPE.
There are various types of CT interactions, based on different possible combinations of molecular orbitals (σ, π, n) and atomic orbitals (p, d) of interacting structures involved in complex formation. For example, the interaction between R3 N and Men+ — a nitrogen-containing organic ligand and a transition metal cation — belongs to the n–d type and is commonly encountered in ligand-exchange and sometimes in ion exchange chromatography. Hydrogen bonds can be considered as the combination of σ–n (for example, -OH and O=) or much weaker σ–π (-OH and Ar) and dipole–dipole interactions. Hydrogen bonding and dipole–dipole interactions are often called "polar interactions", which play a leading role in normal-phase (NP) chromatography. "Dispersive interactions", which are widely found in chromatography, implies non-specific π-interactions, for example, between the alkyl chains of the analyte compounds and the non-polar stationary phase.
π-interactions always play an important role if the interacting molecules have unsaturated or aromatic moieties. For example, it is the relatively weak intermolecular π–π interactions which cause the higher than expected melting point of benzene and strong π–π interactions are responsible for 2,4,6-trinitrophenol complexes with polyaromatic hydrocarbons, such as pyrene. Very strong intramolecular π–d interaction acts as binding force within the ferrocene molecule. Complexes of silver (Ag) and olefins are another example of π–d type interactions.
Different Types of π-Interactions in CT LC
The idea of using CT interactions for separation of unsaturated and aromatic compounds appeared in the 1950s both in charge transfer liquid chromatography (CT LC)1,2 and charge transfer gas chromatography (CT GC). However, these methods were not intensively developed until the 1970s and 1980s.
In the 1980s CT HPLC arose as an extremely effective tool for separating chiral compounds containing aromatic moieties.3 As a rule, retention mechanisms for the most chiral stationary phases (CSPs) are complex because chiral recognition requires (at least) three-point interactions between the chiral selector and enantiomers to be resolved. CT interactions, combined with some "polar" interactions present the major mode of retention on the Pirkle brush-type phases4,5 and CSPs based on silica coated with cellulose derivatives.6
In these instances, where the analytes are non-polar and have strong conjugated π-systems in their chemical structures, it is more appropriate to define the separation as "CT mode" rather than "NP mode". Similiarly, if the analytes are polar and have no unsaturated moieties, the term NP mode is preferred.
There are modern HPLC stationary phases which provide retention purely due to π-interactions when used in combination with non-polar (hexane-based) mobile phases. For example, polyaromatic hydrocarbon (PAH)-coated silica phases separate fullerene, fullerene derivatives and polychlorinated biphenyls (PCB) congeners mostly because of π–π interactions7 and graphitized carbons separate PCB congeners under the same conditions due to the same principle.8
In our experiments, hypercrosslinked neutral polystyrene packing was used in combination with hexane-based eluents to demonstrate the leading role of π-interactions in retention and separation of various aromatic compounds in CT mode. The basic principles of CT HPLC were also established, and used to develop a number of useful applications.6,9
As for π–d interactions, the most pronounced example of their application in LC is the separation of oil and fat triglycerides on silica phases coated with AgNO3.10 Unsaturated and aromatic compounds can also be retained and separated on columns packed with palladium (Pd) or platinum (Pt) particles using hexane as the mobile phase.11 Saturated, monoaromatic, diaromatic and unsaturated compounds of petroleum can be separated from each other using silica coated with Ag complexes.12
Hypercrosslinked Polystyrene
Hypercrosslinked polystyrene was developed by Davankov and co-workers13 and over the past decade polymeric materials of this type have gained great popularity as modern adsorbents for SPE. Examples of materials with properties that can be attributed to hypercrosslinked polystyrene-divinylbenzene (PS-DVB) materials include Purosep 200 and Purosep 270 (Purolite, UK), LiChrolut EN (Merck, USA), Isolute ENV+ (IST, UK) and StrataX (Phenomenex, USA).
Rigid, hypercrosslinked open networks have an extremely high apparent inner surface area (up to 1000–1500 m2 /g) and an almost identical uptake of both polar and non-polar solvents, which explains their excellent compatibility with all mobile phases, from hexane through to methanol and water.
Figure 1 shows computer models of two neighbouring fragments of hypercrosslinked polystyrene, which represent the two smallest possible unstrained cycles composed of six styrene units each. Larger cycles, including interpenetrating and/or mutually condensed cycles, were formed under conditions of statistical post-crosslinking of the initial polystyrene chains by numerous methylene links between phenyl rings. All these elements comprise a rigid open-network structure accessible to low-molecular-weight analytes in any liquid phase.
Although we don't know exactly how this network looks, determination of the pore distribution diagrams suggests the most widespread "molecular pores and channels" of hypercrosslinked polystyrene to be 2–4 nm in size. This is very close to the dimensions of the previously mentioned "smallest possible" unstrained cycles of the hypercrosslinked network. The size of these cycles is comparable with the size of small analyte molecules as chloroform, benzene and anthracene (also shown on Figure 1). This demonstrates why the whole interior of the hypercrosslinked polystyrene bead is accessible to small analytes but not accessible to larger molecules and how all the aromatic rings of polystyrene remain accessible to the analytes.
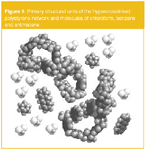
Figure1
Actually, there are two types of hypercrosslinked polystyrene SPE sorbents. Microporous materials such as Purosep 270 have only "molecular pores" whereas the bi-porous material Purosep 200 has "molecular pores" and in addition very large transport channels, or macropores, about 200 nm in diameter. The surface area of these macropores is only about 10–20 m2 /g and, therefore, they do not contribute much to the sorption capacity. However, they do readily assist mass transfer.
In polar solvents hypercrosslinked polystyrene works in a similar way to strongly hydrophobic reversed-phase (RP) packing. In non-polar solvents it works in CT mode and in moderately polar solvents the chromatographic mode is mixed (RP–CT).6
The eluotropic rows of solvents for CT and NP modes are basically different. In the instance of CT mode the elution strength of a solvent also depends on the polarity of the test-analyte in respect of which it is measured. Accordingly, two slightly differing eluotropic rows may be distinguished for non-polar analytes such as naphthalene and for more polar ones such as nitrobenzene (Figure2).14
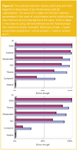
Figure2
In both NP- and CT-mode separations on hypercrosslinked polystyrene, the elution strength is minimum for hexane and other saturated hydrocarbons and maximum for dichloromethane and other halogenated hydrocarbons. However, as opposed to normal-phase mode, in CT mode the elution strength of alcohols is relatively low (because the C-Cl group enters π-interactions much more readily than C-OH), and the elution strength of aromatic solvents is relatively high. This becomes particularly evident when working with non-polar analytes such as PAHs.
Isolation of PAHs from Complex Matrices
There are two common approaches to isolate polyaromatic hydrocarbons from a complex matrix that contains fat. The first procedure is usually used in combination with GC–MS analysis because it provides cleaner samples. It includes the following steps:
i) Hydrolysis of fatty sample and dilution of the alkaline hydrolysate with water.
ii) Extraction of diluted hydrolysate with hexane (cyclohexane).
iii) Clean-up of hexane extract using polar SPE adsorbents.
This method is time- and labour-consuming and, therefore, has a very low sample throughput.
The second approach is often used in combination with HPLC with fluorescence detection:
i) Extraction of a sample with hexane or cyclohexane.
ii) Re-extraction of the hexane solution with acetonitrile.
This approach provides much higher throughput, but has obvious limitations. If the fat content is high, it is rather hard — or even impossible — to separate the hexane and acetonitrile layers from each other at the second stage, even by centrifuge. Additionally, the extracts obtained using this procedure can contain large amounts of contaminants. This can be problematic when concentration of the analytes by evaporating the solvent to dryness and re-dissolving the residue in the same or another solvent of smaller volume is needed to achieve the required sensitivity.
A possible alternative to these two approaches is SPE of PAHs from hexane extracts using hypercrosslinked polystyrene SPE phases. This procedure can include two or three stages depending on the final analytical method used (stages i and ii for high performance liquid chromatography with fluorescence detection (HPLC–FLD) and stages i, ii and iii for gas chromatography mass spectrometry (GC–MS):
i) Extraction of a sample with hexane or cyclohexane.
ii) SPE on hypercrosslinked polystyrene (including the steps of washing the cartridge with hexane and eluting PAHs with dichloromethane).
iii) Evaporating the dichloromethane extract to dryness, dissolving the solid residue in hexane and re-extraction of PAHs with acetonitrile.
Procedures i and ii for HPLC–FLD employed a cartridge with 500 mg of Purosep 200 (Purolite, UK). HPLC separation of the SPE eluate was achieved using the specially developed Wakosil II C18 AR (250 mm × 4.6 mm) kindly provided by SGE Analytical Science (Australia). Figure 3 shows the chromatogram of a 16-component PAH test mixture together with the chromatogram of an extract of a smoked fish sample obtained according to the procedures described in i and ii.
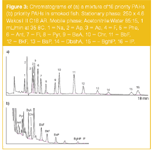
Figure3
This type of SPE-HPLC(–FLD) approach provides a very high throughput method with acceptable recoveries of important PAHs, typically between 80% and 99% for PAHs in edible oils and fats. However, we have observed a 10–15% drop in recoveries for some fatty food samples, which is probably due to overloading of the SPE cartridge with matrix compounds, but can be taken into account through matrix matching of calibration standards.
Simple Clean-up for the Analysis of PCB and PCDF Congeners
The determination of polychlorinated biphenyl (PCB), polychlorinated dibenzodioxin (PCDD) and polychlorinated dibenzofuran (PCDF) congeners in soil, water and bioassays is one of the most important analyses in ecological monitoring. To achieve the lowest possible limits of detection (LOD) with the use of GC–MS, contaminants such as humic acids and hydrocarbons for soil and water samples and triglycerides and peptides for bioassays samples should be selectively removed during the SPE sample preparation step.
We have achieved this by developing a very simple clean-up procedure, which is based on SPE on microporous hypercrosslinked polystyrene (Purosep 270). A hexane extract of the soil or ground water sample is concentrated by evaporating the solvent under a stream of nitrogen until the final sample volume was around 0.2 mL. The concentrated sample was then introduced onto the microporous hypercrosslinked polystyrene SPE cartridge, which had been pre-conditioned with hexane. The loaded cartridge was then washed with several portions of hexane to remove high-molecular-weight interferences and the analytes of interest were then eluted sequentially using hexane–dichloromethane mixtures according the scheme given in Table 1.
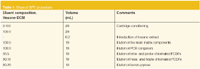
Table 1: Steps of SPE procedure
The microporous hypercrosslinked polystyrene works here as a RAM (restricted access material) adsorbent that (due to the size exclusion effect) remains inaccessible for polymeric and even large monomeric molecules. Thus, humic acids, peptides, saturated hydrocarbons and triglycerides are washed out from the cartridge with the first portion of hexane. The second hexane fraction contains all the PCBs.
PCDFs have more conjugated aromatic systems and are, therefore, more strongly retained on the SPE phase and to elute these from the cartridge more polar 95:5 and 90:10 hexane–dichloromethane mixtures have to be employed. Similarly, higher degrees of conjugation, such as penta-cyclic PAHs such as benzo[α]pyrene require an 80:20 hexane–dichloromethane mixture for complete elution.
This method was achieved using a cartridge containing 500 mg of Purosep 270 (Purolite, UK) with GC separation on the specifically developed capillary columns BPX-PCB, BPX-Dioxin I and BPX-Dioxin II [SGE Analytical Science (Australia)]. Typical chromatograms are available from reference 15.
Determination of Furan Derivatives in Transformer Oil
An important HPLC application for the power-generating industry is the analysis of four furan derivatives (5-hydroxymethylfurfurol, furfurol, 2-acetylfuran and 5-methylfurfurol) in mineral transformer oil. These compounds are the degradation products of cellulose-based electrical insulation materials used in transformers and these compounds are useful as specific markers for insulation diagnostics. The common approach employs LLE of the transformer oil with acetonitrile or acetonitrile–water mixtures. This method is not always applicable as the partition coefficients and consequently the analyte recoveries, vary depending on the origin and even the batch of the oil.
A simple alternative SPE procedure using hypercrosslinked polystyrene was developed to solve this problem and includes the following steps:
i) Dilution of an oil sample with hexane.
ii) SPE on hypercrosslinked polystyrene (including washing the cartridge with hexane, drying the cartridge with nitrogen and eluting analytes with acetonitrile–water 1:1 mixture).
This SPE method was achieved using a cartridge containing 500 mg of Purosep 200 (Purolite, UK). Recoveries of furan derivatives according to this procedure vary between 95% and 98%, except for 5-hydroxymethylfurfurol (75–80%). A typical HPLC chromatogram obtained using this method for the analysis of a transformer oil containing 30 ppb of each furan derivative is shown on Figure 4.
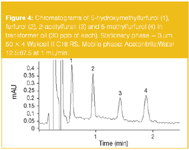
Figure5
Large-scale Applications for Oil Refining
SPE is not the only field where adsorption resulting from π-interactions can be used. Hypercrosslinked polystyrene materials working in the CT mode can be effectively used in large-scale industrial adsorption techniques. There are currently several types of neutral hypercrosslinked polystyrene resins produced by Purolite (UK) and Bayer AG (Germany) for the needs of industry. These materials are spherical and monosized (this means that they have very narrow particle size distribution). In our opinion, selective adsorption can find many applications in the production of fine fuel and mineral oils.
For example, one of the key stages in oil refining is to remove sulphur-containing aromatic compounds, such as benzothiophenes and dibenzothiophenes, from the gasoline. Our preliminary experiments have shown that chemical modified hypercrosslinked polystyrenes can give rise to a new generation of adsorption materials possessing very high capacity and improved adsorption selectivity towards various heterocyclic aromatic compounds. Thus, it is possible to develop a variety of resins for the selective clean-up of fuel and mineral oils from the residues of different unsaturated compounds such as unsaturated hydrocarbons and dibenzothiophenes.
Conclusion
Hypercrosslinked polystyrene is characterized by a unique rigid open-network structure with high density of alkyl-substituted benzene rings that are exposed to relatively small (below 500 Da) solutes. By using π-type interactions, these adsorbing materials can selectively retain aromatic compounds, heterocyclic compounds and to a lesser degree, molecules with C=C, C=O, C=N double bonds from non-polar media (e.g., hexane extracts). This property of the polymer provides options for the selective extraction and pre-concentration of such aromatic and polar organic compounds from non-polar matrices, such as oils, fats, hexane-extracts and so on. Using hypercrosslinked polystyrene as an SPE-material allows the development of rapid, selective and sensitive techniques for the analysis of many contaminants in natural and mineral oils, for food and environment monitoring and for the selective extraction from physiological fluids of lipophilic species that are capable of entering π-interactions. Additional benefits of hypercrosslinked polystyrene SPE phases include high recovery values, the possibility of reusing the SPE cartridge and exploiting its ability to exclude large-molecular-weight components of the sample matrix.
Constantin S. Sychov obtained his PhD in chemistry in Moscow, Russia in 2004 and currently works as an independent marketing specialist, consultant and technical writer. His research interests include the development of HPLC and SPE applications, development and investigation of novel materials for adsorption technologies, writing educational literature, development of approaches for studying intermolecular interactions, particularly in the field of CT and HILIC liquid chromatography.
Vadim A. Davankov obtained his PhD degree (1966) and then Doctor of Science degree (1975) from the Nesmeyanov-Institute of Organoelement compounds (Russian Academy of Sciences) in Moscow. Since 1975 he has been head of the Laboratory for Stereochemistry of Sorption Processes at the same institute and he became a full Professor in 1980, Distinguished Scientist of the Russian Federation. He was the first to introduce chiral ligand exchange chromatography and then hypercrosslinked-type polymeric adsorption materials and he was the recipient of the international golden Chirality Medal (1999) in stereochemistry and the Gold Martin Medal (2005) in chromatography.
Natalia A. Proskurina graduated from the Samara State University and is currently completing her PhD thesis on application of hypercrosslinked polystyrene in the capacity of solid-phase extraction material at the Nesmeyanov-Institute of Organoelement Compounds in Moscow, Russia.
Alyona Ju. Mikheeva graduated from the St-Petersburg technological university and is currently completing her PhD thesis on development of approaches to the analysis of priority environmental pollutants at the Water Control and Research Center in St-Petersburg, Russia.
Acknowledgements
C.S.S. wishes to gratefully acknowledge SGE Analytical Science for its generous support for this work.
References
1. M.S. Newman and D. Lednicer, J. Amer. Chem. Soc., 78, 4765 (1956).
2. F. Mikes, G. Boshart and E. Gil-Av, J. Chromatogr., 122, 205 (1967).
3. W.H. Pirkle, C.J. Welch and M.H. Huin, J. Chromatogr., 607, 126–130, (1992).
4. Y.H. Kim, Chiral Recognition in Liquid Chromatography through Charge Transfer Complexation: Its Mechanism and Aplications. Thesis for the degree of PhD. Weizmann Institute of Science, Rehovot, April 1981.
5. L. Oliveros et al., J. Chromatogr., 543, 277–286 (1991).
6. C.S. Sychov et al., J. Chromatogr. A, 1030, 17–24 (2004).
7. K. Kimata et al., J. Chromatogr. A, 786, 237–248 (1997).
8. K.R. Echols, J. Chromatogr. A, 811, 135–144 (1998).
9. V.A. Davankov et al., J. Chromatogr. A, 987, 67–75 (2003)
10. P.J.W. Schuyl et al., J. Chromatogr. A, 810, 53–61 (1998).
11. B.J. Bassler et al., J. Chromatogr., 461, 139–147 (1989).
12. G. Felix et al., J. Chromatogr., 461, 347–352 (1989).
13. V.A. Davankov and M.P. Tsyurupa, React. Polym., 13, 27 (1990).
14. C.S. Sychov. Application of Hypercrosslinked Polystyrene in SPE and HPLC. Thesis for the degree of PhD. Institute of Physical Chemistry, Moscow, December 2004
15. TP-0112-C.pdf, TP-0093-C.pdf.
Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.





