Frequently Asked Questions about Hydrogen Carrier Gas
Chromatographers are choosing hydrogen carrier gas more often for its performance and, in the face of increasing helium prices, cost. In this instalment of "GC Connections", John Hinshaw addresses some of the more frequently asked questions he encounters regarding hydrogen as a carrier gas.
Many gas chromatographers are considering hydrogen carrier gas instead of helium or nitrogen, but they might hesitate to do so because they have questions about performance, safety, or cost. This month's "GC Connections" focuses on these and other issues related to hydrogen in the gas chromatography laboratory.
Performance
For sheer column performance, hydrogen carrier gas offers some strong advantages over helium or nitrogen. Hydrogen yields higher plate numbers at rapid linear velocities and achieves higher velocities at lower pressures. At the same time, the presence of extra hydrogen can affect flame ionization and other detectors that use hydrogen fuel gas.
Does hydrogen carrier gas affect retention times? The answer is yes and no. Retention times in gas chromatography (GC) are controlled by several factors: the distribution coefficient (K) of a solute in the column, which is not affected by the choice of carrier gas; the column dimensions of length, inner diameter and stationary film thickness, all of which we will keep constant in this discussion; and the average carrier gas linear velocity (ū). As the linear velocity increases, isothermal retention times decrease in exact proportion, so doubling the velocity cuts retention times in half. We will look at the effects of changing carrier gas for three situations: constant velocity, constant inlet pressure and constant flow-rate and then we will see what happens if the column is temperature programmed.
In addition to its dependencies upon the column dimensions and pressure drop, the linear velocity is influenced by the viscosity of the carrier gas. As many readers will know, hydrogen is a bit less than half as viscous as helium or nitrogen at the same temperature, as shown in Figure 1, and so for this reason hydrogen requires a lower pressure drop to achieve the same average carrier gas velocity as for helium or nitrogen. For example, a 50 m × 250 μm column will deliver an average velocity of 60 cm/s at 100 °C with 58.6 psig (404 kPa) of helium or with 27 psig (186 kPa) of hydrogen; the situation is similar for nitrogen. If the velocity is unchanged with a hydrogen versus helium carrier, then retention times remain the same too, while the inlet pressure that is required with hydrogen will be about half as much as with helium.
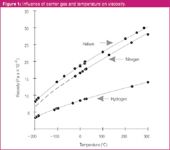
Figure 1
Conversely, if the same inlet pressure were applied with hydrogen as with helium, then the hydrogen carrier gas would cause peaks to be eluted in less time, because the linear velocity would be faster than with helium. In the previous example, helium at 27 psig would have an average linear velocity of 29.4 cm/s and so with hydrogen carrier at the same pressure, all of the peaks' retention times would decrease in the proportion 29.4/60 ≈ 0.5. The relationship between linear velocity and pressure drop is somewhat nonlinear because of effects stemming from the compressibility of the carrier gas, but in general, the effect of switching from helium to hydrogen on retention time will be to cut retention times roughly in half if the inlet pressure is unchanged.
At constant flow-rates, the situation is intermediate between the effects at constant velocity and those at constant pressure. At 27 psig of helium, the column flow-rate is going to be 1.43 cm3/min and the average velocity will be 29.4 cm/s. The hydrogen carrier pressure required for the same flow-rate will be about 16.3 psig (112.4 kPa), but now the average velocity will increase to 37.5 cm/s. Therefore, in this example, retention times will decrease by a factor of 29.4/37.5 = 0.78 for hydrogen compared with helium at the same column flow-rate.
In summary: for isothermal operation at constant average linear velocity, retention times are not affected by changing the carrier gas. At constant inlet pressure hydrogen carrier will cause peaks to be eluted in about half the time and at constant flow-rate retention times will be about 78% with hydrogen compared with helium.
What if column temperature programming is used? If a constant linear velocity can be maintained during the temperature programme by an electronic pneumatic system, then retention times will not change, but this feature is not available in all pneumatic systems. At constant inlet pressure, hydrogen will cause peaks to be eluted earlier, but their elution temperatures will also be reduced and this can change the relative retentions of peaks with divergent chemical characteristics, such as hydrocarbons compared with polar compounds. The same effects will occur, but to a lesser degree, when comparing hydrogen with helium at constant column flow-rate. In general, if the column is temperature-programmed then each peak should be reidentified if the carrier gas is changed and it might be a good idea to optimize the temperature programme ramp rate by increasing it to restore, as closely as possible, the elution temperatures that were obtained with the helium carrier.
How can I manage hydrogen fuel flow in my flame ionization detector with hydrogen carrier gas? Flame ionization detection (FID) sensitivity depends upon the flows of hydrogen and air fuel gases, make-up gas and the flame jet inner diameter. The simple answer is to reduce the hydrogen fuel gas flow so that the total hydrogen flow through the detector remains close to the manufacturer's specified optimum flow — usually between 40 and 50 cm3/min. This approach works with all pneumatic systems as long as the column flow remains constant throughout the chromatographic run.
What happens when the column oven temperature changes during the run? The column flow-rate will go down as the temperature increases if the carrier gas pressure is held constant. For narrow-bore columns (under 320 μm i.d.) starting at average linear velocities under 50 cm/s, the temperature-induced flow change might be small enough that the total hydrogen flow through the flame ionization detector will remain within the optimum range (about ± 2.5 cm3/min). With wide-bore columns (320 μm i.d. or larger), or at higher initial linear velocities, the flow can drop sufficiently during temperature programming to affect FID sensitivity significantly.
I recommend the use of electronic pneumatics in all instances with hydrogen carrier gas, for a number of reasons. Most electronic pneumatic systems have the capability of linking the flame ionization detector and carrier gas hydrogen flows and adjusting the fuel gas flow so that the total hydrogen flow-rate remains constant during temperature programme ramps while the column flow changes. Alternatively, chromatographers can use constant carrier gas flow mode with electronic pneumatics and maintain constant FID hydrogen flow as well.
What should I use for detector make-up gas if I use hydrogen carrier gas? Detector make-up gas serves two main purposes. First, it flushes any dead volume inside a detector to reduce extra band broadening at low capillary column flow-rates. Some detector designs use the hydrogen fuel flow as a make-up flow too — these detectors do not require additional make-up gas. Make-up gas also increases the velocity of the hydrogen–carrier gas mixture passing through the flame jet and helps increase sensitivity and linearity. Consult your GC system user's manual for exact recommendations on combinations of column inner diameter, column type and FID jet configuration.
If your flame ionization detector requires an additional make-up gas supply beyond the normal hydrogen fuel gas flow, you should always use an inert gas and not more hydrogen — the total hydrogen flow-rate must equal the optimum flow, not just the combination of hydrogen fuel and hydrogen carrier. Most detectors call for make-up gas flows around 30 cm3/min, so along with 2–5 cm3/min or more of hydrogen carrier, there is little overhead for hydrogen fuel flow and hydrogen make-up gas; turning the fuel flow off is not an option.
Either helium or nitrogen will perform well as make-up gas with hydrogen carrier. The make-up gas purity must be the same as the carrier gas to avoid increasing detector noise unnecessarily. When switching from helium to hydrogen carrier with an FID-equipped instrument, plan on using the new hydrogen supply both for the detector and the carrier gas. This way, the total number of gas supplies will remain at three — hydrogen carrier + FID hydrogen, helium or nitrogen make-up gas and FID air.
Does hydrogen deliver higher plate numbers or separation efficiency at high separation speeds? The carrier gas influences column efficiency, or plate number, in a couple of ways. The theoretical optimum linear velocity is influenced directly by solutes' gas–gas diffusion rates — higher diffusion will increase the optimum velocity. For example, the gas–gas diffusion rate of n-octane in helium at 130 °C is 0.383 cm2/s, while in hydrogen it increases to 0.467 cm2/s, therefore, we would expect the optimum velocity to increase with hydrogen carrier compared with helium. This is the case, as shown in Figure 2, where the optimum for hydrogen is almost 40 cm/s, while for helium the optimum is closer to 30 cm/s. The minimum theoretical plate heights for hydrogen and helium are close to each other, however, and so at the optimum linear velocity little difference in the theoretical plate number would be expected for significantly retained peaks.

Figure 2
What is interesting about hydrogen is that it behaves better at higher velocities than does helium. In Figure 2, the solid portions of each plotted line represent a useful linear velocity range over which the plate height stays within 25% of the optimum value. This equates to a maximum loss of resolution between adjacent peaks of about 12%. For helium, this range is from 18 to 45 cm/s, while for hydrogen it ranges from 25 to 65 cm/s. The ratio of the highest to the lowest velocity across this range is roughly the same for helium and hydrogen — about 2.5:1 — but hydrogen works better at higher velocities and covers a wider range of velocities while remaining close to optimum. If a chromatographer wanted to push the speed of a separation by increasing the linear velocity, then hydrogen is a logical choice. At 60 cm/s, using the data in Figure 2, hydrogen produces about 33% more plates than would helium at the same velocity, so hydrogen performs better at high speeds.
Why not use nitrogen instead of helium or hydrogen? Nitrogen (the "other" carrier gas) has a minimum plate height that is about 10% lower (better) than either helium or hydrogen, see Figure 2. This means that if we were to operate at the optimum velocity, then nitrogen would deliver around 10% more theoretical plates. The effect on peak resolution is negligible, however, because resolution is related to the square root of the plate number. What is not so good about nitrogen is the much narrower velocity range over which the plate height remains close to optimum, from about 8 to 20 cm/s in this case. The optimum linear velocity depends upon the solute and temperature, so for different solutes, and as the temperature changes during programming, the optima for each solute will appear at velocities somewhat different than the theoretical minimum. With nitrogen it is more likely that, for a given solute, the plate height will be farther from optimum than with carrier gases such as helium or hydrogen for which the plate height does not depend as strongly upon the linear velocity.
Will hydrogen react with my unsaturated or aromatic solutes? Hydrogen is a reactive compound that will hydrogenate unsaturates and aromatics under the influence of temperature, pressure and a catalyst. Sufficient conditions for hydrogenation do not exist inside conventional wall-coated fused-silica capillary columns, but the use of nickel columns with hydrogen carrier gas at higher temperatures might better be avoided if reactive solutes are to be separated.
How pure does hydrogen carrier gas need to be? Hydrogen carrier gas should be of the same purity level as helium carrier — "research" grade, or 99.9999% purity for trace work under 1 ppm, or at least 99.9995% for most normal analyses. Hydrogen generators fall into two broad groups, depending upon the type of generation mechanism. One type is better suited as a carrier gas supply because it delivers higher purity hydrogen and the other is better suited for fuel gas use. The manufacturer's literature will specify the purity and suitable applications.
Do I need to filter hydrogen carrier gas from a generator or a tank? Hydrogen carrier is just like any other high-purity carrier gas and requires the same types of filters to be installed close to each supplied GC system. The filters are not needed to purify hydrogen as it leaves the generator or tank, they are needed to trap any contaminants introduced from regulators, connecting tubing and fittings, as well as to isolate multiple instruments from each other.
If I use a hydrogen generator, will there be water or oxygen in the carrier gas? Ultrahigh-purity hydrogen carrier gas generators remove water and the oxygen that is formed by passing the generated hydrogen through a palladium membrane that prevents passage of substances other than hydrogen.
Do hydrogen generators need specifically purified water and chemical consumables? Yes. Hydrogen generators use deionized water that will need to be refilled regularly. Continuous flow water supplies are also available. Some carrier-gas grade generators will need periodic replacement of a deionizer bag, but normally this only needs to be done once or twice a year. Refer to the product manual for exact maintenance requirements.
How much hydrogen flow does a GC system consume? A single-channel GC system with FID and split–splitless injection will consume 100–500 cm3/min of hydrogen carrier and fuel gases, depending upon the split flow-rate and column consumption. Gas-saver operation — turning off the split flow when not required — will reduce carrier gas consumption considerably. In general, a hydrogen generator should run at under 80% of its rated capacity, so for example, a generator capable of delivering 1 L/min could operate two single-channel GC systems with split flows up to 300 cm3/min each, or possibly three GC systems at lower split flows. It is better to over-specify a generator and, thus, be sure not to run out of generating capacity for future planned applications.
I already have hydrogen cylinders that supply detector fuel gas in my laboratory: Can I use these for carrier gas as well? Hydrogen for detector fuel gas might not be pure enough for carrier gas use. However, UHP or research-grade purity hydrogen cylinders or generators can be used for fuel gas as well.
Safety
Most GC laboratories now use flame ionization or other detectors, such as flame-photometric or nitrogen–phosphorus types, that consume hydrogen as a fuel gas. If your laboratory now uses or plans to use hydrogen as fuel or carrier gas, you should review safety equipment and procedures related to the use of hydrogen. It is also a very good idea to consider the hazards of nonflammable compressed gases such as nitrogen, air and helium. Consult with instrument and hydrogen generator manufacturers, local safety authorities. Many companies will have a safety policy that must be adhered to as well. In all instances, prepare the laboratory suitably before introducing any flammable, compressed, or otherwise hazardous gases. I strongly recommend the use of electronic carrier gas controls because such automated systems will detect many potentially unsafe fault situations that could result in leakage and will shut off carrier gas flow.
I will put in a good word for hydrogen generators here: a hydrogen generator stores only a small amount of hydrogen. The exact amount depends upon the generator capacity but typically is less than 0.20 L. Compared with the 6000–8000 L of gas that is stored in a full-size laboratory gas cylinder when new, it should be obvious that a hydrogen generator presents a considerably lower hazard in terms of the amount of stored flammable gas. Even so, this does not change the rate and total amount of hydrogen that is consumed nor does it remediate the hazards of using flammable gases, it just minimizes the total amount that is present in the laboratory at any one time.
Cost
What is the cost of purchasing or leasing a hydrogen generator, compared to using cylinders? The cost of a hydrogen generator, when used to replace helium carrier gas, will pay for itself in a relatively short time. It is not possible to review exact purchase-versus-lease figures or return on investment payback times in this article: these will vary considerably depending upon individual circumstances and usage, and on future helium costs.
Where does helium come from, anyway? Why is the price of helium going up so much? Helium exists in the air at around 50 ppm, too low a level for practical purification. The natural decay deep underground of radioactive elements such as uranium to alpha particles results in the formation of helium, which then mingles with natural gas deposits at concentrations as high as 2–5%. Helium is fractionated cryogenically from natural gas and stored. For many years, the US produced 90% or more of the world's helium. In 1996, the US began a programme of privatizing its helium reserves and liquidating its helium supplies, which has resulted in an increasing shortage of helium. The demand for helium has increased considerably since 2000, outstripping supply and with few new helium sources becoming available recently, the price has increased concomitantly.
Conclusion
Hydrogen is a very viable alternative to helium carrier gas for capillary column GC. Hydrogen yields better column performance at lower inlet pressures and across a wider linear velocity range and it can be considerably less expensive than helium. Carrier-gas grade hydrogen generators provide a convenient means to produce very high purity hydrogen on demand. The safety issues of working with hydrogen as carrier and fuel gas can be addressed in the laboratory through compliance with federal, state and local standards and with internal safety policies and procedures. Chromatography instrument manufacturers, gas suppliers and hydrogen generator producers are able to provide guidance and answer most questions about the use of hydrogen in the chromatography laboratory.
"GC Connections" editor John V. Hinshaw is senior staff engineer at Serveron Corp., Hillsboro, Oregon, USA and a member of the Editorial Advisory Board for LCGC Europe. Direct correspondence about this column should be directed to Alasdair Matheson e-mail: amatheson@advanstar.com
For an ongoing discussion on GC issues with John Hinshaw and other chromatographers, visit the Chromatography Forum discussion group at www.chromforum.com.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.











