Understanding How Dwell Volume Can Affect Selectivity in Reversed-Phase Gradient Chromatography
LCGC Europe
The effect of dwell volume on chromatographic selectivity can be successfully modelled using retention prediction software. Hence, the robustness of reversed-phase LC gradient methodologies, with respect to dwell volume, can be conveniently assessed.
Image Credit: tomozina1/stock.adobe.com/Andy Garside

Relatively small disparities in dwell volume, that is, the typical differences observed between ultrahigh-pressure liquid chromatography (UHPLC) and standard LC instrumentation, have been shown to have a profound effect on reversed-phase gradient chromatographic selectivity. These anomalous effects can be circumvented by either the addition of an isocratic hold (translating from a high to low dwell volume) or by performing a delayed injection (translating from a low to high dwell volume) after the gradient has been started. These selectivity discrepancies can be observed if the analytes are not highly retained at the column inlet during the initial isocratic phase of the gradient corresponding to the dwell volume. The effect of dwell volume on chromatographic selectivity can be successfully modelled using retention prediction software. Hence, the robustness of reversed-phase LC gradient methodologies, with respect to dwell volume, can be conveniently assessed.
Differences in the dwell volume and column volume ratio (Vd/Vm) between dissimilar liquid chromatography (LC) instruments can profoundly affect the resultant chromatographic selectivity of reversed-phase separations (1–3). This is of major importance for chromatographers who receive or transfer gradient LC methodologies between laboratories where the dwell volumes of differing LC instrumentation may not be similar (assuming that the column dimensions are kept constant). This has been exacerbated with the introduction of ultrahighâpressure liquid chromatography (UHPLC) instruments (with reduced dwell volumes compared to traditional LC instrumentation) into both research and quality control and operation departments in recent years. While there are numerous seminal academic publications (3–5) relating to this phenomenon, this article discusses and addresses, in nonmathematical terms, the practical importance of the problem of transferring between LC instruments possessing dissimilar dwell volumes.
Experimental
Experimental work was performed on a Nexera X2 UHPLC system (Shimadzu) equipped with LC-30AD pumps, DGU-20A5R degassers, SIL-30AC autosampler, CTOâ20AC column oven, and SPD-M30A photodiode array detector (PDA) equipped with a 1 μL/10-mm pathlength flow cell and 40-μL mixer (Shimadzu UK Ltd). The system was controlled and data collected by means of LabSolutions software (Shimadzu UK Ltd, version 5.86). A 50 × 2.1 mm, 1.7-μm Agilent Eclipse XDB-C18 column was used in the study (Agilent Ltd). Modelling was performed using ACD Lab’s LC Simulator (version 2016.2.2). Unless otherwise stated, the following chromatographic conditions were employed: 0.75 mL/min; 40 °C; detection at 254 nm; mobile phase A, 0.1% v/v formic acid in water and mobile phase B, 0.1% v/v formic acid in acetonitrile. Dwell volume modifications were simulated by the insertion of isocratic hold times prior to commencing the gradient.
Results and Discussion
This article examines the importance of differing dwell volumes on the gradient reversed-phase chromatographic selectivity of two mixtures containing hydrophilic and hydrophobic components, respectively. Approaches to circumvent dwell volume differences will also be addressed. In addition, retention modelling software will be evaluated to predict the effect of dwell volume variations on these separations. The importance of retaining the most polar analyte at the column inlet during the initial isocratic phase, equivalent to the dwell volume, will be highlighted in an approach to describe selectivity in gradient chromatography. Finally, in pictorial terms, why changing the dwell volume can have the potential to affect chromatographic selectivity will be discussed.
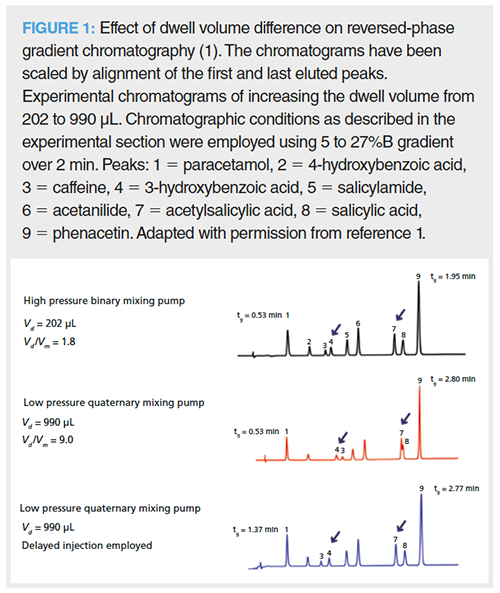
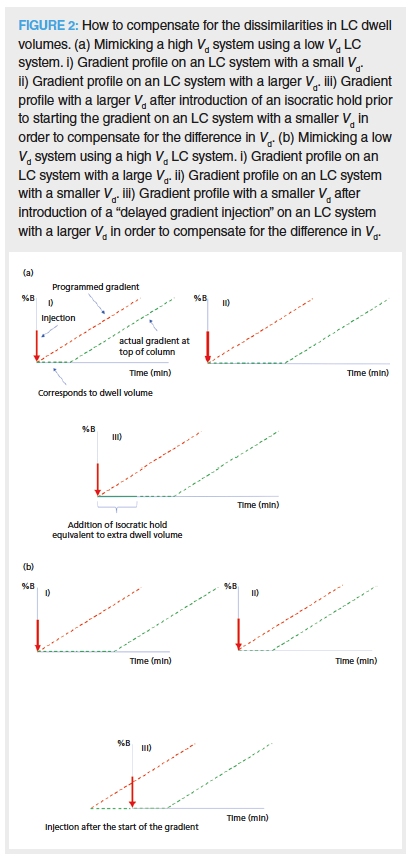
How to Rectify the Problem: The potential of differing dwell volumes to affect chromatographic selectivity in gradient reversed-phase LC is highlighted in Figure 1, where there is a complete elution reversal between analytes 3 and 4 when transferring between a binary high-pressure LC system (Vd = 202 μL, Vd / Vm = 1.8) and a quaternary low-pressure LC system (Vd = 990 μL, Vd / Vm = 9.0) (1). This can be rectified by performing a delayed injection, that is, by injecting the analytes after the gradient has been started to remove the excess dwell volume on the low-pressure LC and large dwell volume instrumentation (Figure 2). Alternatively, a translation in the opposite direction can be rectified by the introduction of an initial isocratic hold. There are software programs on the internet that can assist the chromatographer in this task (1,6). In addition, most of the major instrument manufacturers now incorporate this type of capability within their data acquisition software.
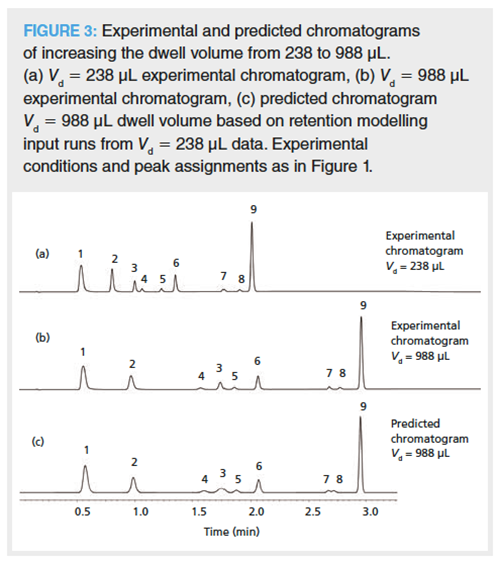
Retention Modelling to Assess the Effect of Dwell Volume Differences: The effect of dwell volume changes on the chromatographic selectivity of reversed-phase separations can be assessed using commercially available retention modelling software. For small molecules this can be achieved using a simple linear model based on only two gradient input experiments (differing gradient time [tG] experiments with the same dwell volume), respectively. The predicted chromatogram for the larger dwell volume mirrored the actual run with an increased dwell volume (achieved by insertion of an appropriate isocratic hold time prior to commencing the gradient) and can be seen in Figure 3. It is also possible to do this for larger molecules, such as proteins, but then a modified model may be required (7).
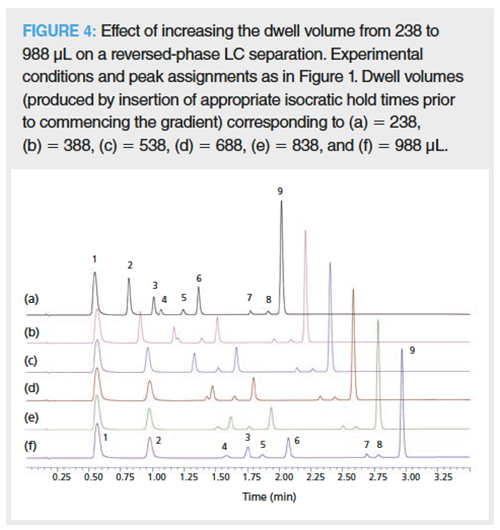
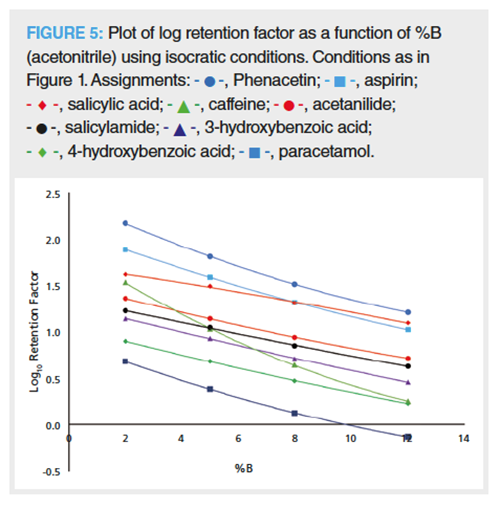
How do Dwell Volume Differences Affect Chromatographic Selectivity in Reversed-Phase LC Separations?: Why do dwell volume variations have the potential to promote such drastic changes in chromatographic selectivity? As can be seen in Figure 4, there is a progression in the change in selectivity between caffeine (3), 3-hydroxybenzoic acid (4), and salicylamide (5), and also between acetylsalicylic acid (7), salicylic acid (8), and phenacetin (9) as the dwell volume is steadily increased. Retention modelling software relies on the linear solvent strength model (3,5,8). Hence, the answer must be dependent on the analyte’s retention as a function of the proportion organic (%B) in the mobile phase at the start of the gradient-the bigger the dwell volume, the longer the analyte will experience isocratic elution conditions. The earliest eluting analyte’s (for example, peak 1, paracetamol) peak width is relatively broad compared to the later eluting peaks, which is because it elutes in the initial isocratic phase corresponding to the dwell volume of the LC system. However, its retention and peak width does not change as the dwell volume is increased, that is, insertion of longer initial isocratic conditions before the gradient “kicks in”. In comparison, later eluting peaks all experienced increased retention as the dwell volume was increased and most experienced increased peak widths (later peaks were less affected than earlier ones) suggesting that they too start to migrate down the column during the initial isocratic periods (corresponding to the dwell volume). The separation between caffeine (3)/3-hydroxybenzoic acid (4) and acetylsalicylic acid (7)/salicylic acid (8) is extremely sensitive to dwell volume differences. Caffeine elutes before 3-hydroxybenzoic acid and acetylsalicylic acid before salicylic acid at low dwell volumes (Vd = 238 μL), whereas at high dwell volumes (Vd = 988 μL) 3-hydroxybenzoic acid elutes before caffeine and salicylic acid before acetylsalicylic acid (Vd = 1976 μL, result not shown). Construction of a log retention factor versus %B plot for the analytes mirrors these observations (Figure 5) in that the elution order between caffeine/3-hydroxybenzoic acid and acetylsalicylic acid/salicylic acid switch at low and high %B isocratic conditions, respectively. At larger dwell volumes the peaks experience the initial isocratic conditions for a longer time, which means that lowly retained analytes will move further down the column. In comparison, at smaller dwell volumes the gradient “kicks in” earlier so that the peaks will experience the higher %B sooner-rationalizing the elution order changes observed on increasing the dwell volume in this gradient example.
Defining Selectivity in Gradient Chromatography: Selectivity in gradient chromatography (α*) has been defined in various publications (3,9) where gradient chromatography presents a more complex situation than isocratic chromatography in that changing eluent composition, dwell volume, column dead volume, and gradient shape can all influence the selectivity. A detailed description of how dwell volume affects retention in gradient chromatography can be found in reference 8, however, no comment on its effect on selectivity was made. A recent paper (9) evaluated a variety of ways of measuring α* using a range of peptides (molecular weight 2–3 kDa). The most robust and intuitive methodology was defined by equation 1, where tg in equation 2 is the retention time in gradient elution and tm and td are column dead time and dwell time, respectively:

It was concluded, however, that the value of α* as described in equation 1 was heavily dependent on the analyte’s gradient elution time, despite having a similar degree of separation. In order to obtain a selectivity measure for samples independent of where a pair of analytes eluted in the gradient, an alternative measure Δtg* was defined (equation 3). Δtg* provided identical selectivity values, regardless of the analyte’s elution time in the gradient. Another advantage of this approach is that it also should compensate for differences in dwell time (td) and column dead time (tm):

Where tg is the retention in gradient elution, tgRef1 is the retention in gradient chromatography of the first eluting compound in the sample, and tgRef2 is the retention of the last eluting peak in equation 4. tg* values between 0 and 1 are generated. This approach was shown to be valid for compensating between LC instruments with different dwell volumes for the gradient analysis of peptides. This approach works well for solutes approximating to a bind and elute (on/ off) retention mechanism because they are highly retained (trapped) at the column inlet during the beginning of the gradient program.
Can This Approach be Applied to Small-Molecular-Weight Analytes?: This same approach, when applied to small polar analytes (logD = -0.34–1.78 at pH 2.7) using the experimental conditions described in Figure 1, failed to compensate for dwell volume differences because nearly all of the peaks to some extent failed to be retained at the column inlet.
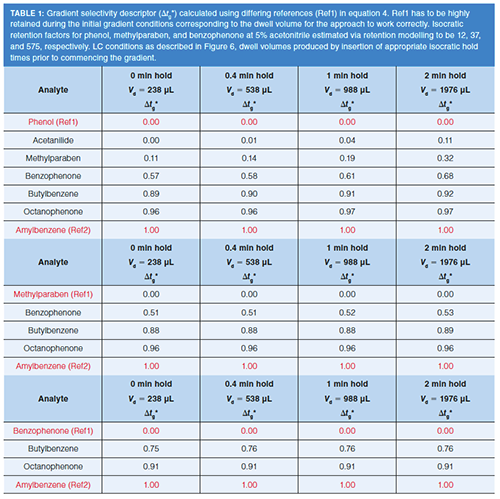
In order to investigate this more thoroughly, a range of more hydrophobic analytes (see Table 1, logD = 1.24–4.75 at pH 2.7), which used a wider gradient range (ΔΦ = 0.9 corresponding to a 90% change in strong solvent) compared to the small polar analytes investigated in Figure 3 (ΔΦ = 0.22), were evaluated. The results shown in Table 1 highlight the fact that for the Δtg* approach to work, the first reference peak (tgRef1) must possess a large enough retention factor (> 40) at the initial %B composition in order for it to be retained at the column inlet; the approach failed to work for acetanilide and phenol (too polar to be fully retained at the column inlet during the initial isocratic conditions), but worked effectively for analytes that were fully retained (more retentive than methylparaben). This further validated the approach of using Δtg* to describe selectivity in the gradient analysis of peptides (9). Peptides are normally highly retained at the initial gradient conditions and respond much more strongly than small molecules to an increase in %B (that is, the slope [S] of the log k versus %B plots are typically >50 for large molecules [S ≈ 0.25 × (MW)0.5 (3)] compared to S values of ≈ 4–5 for small molecules <1 kDa).
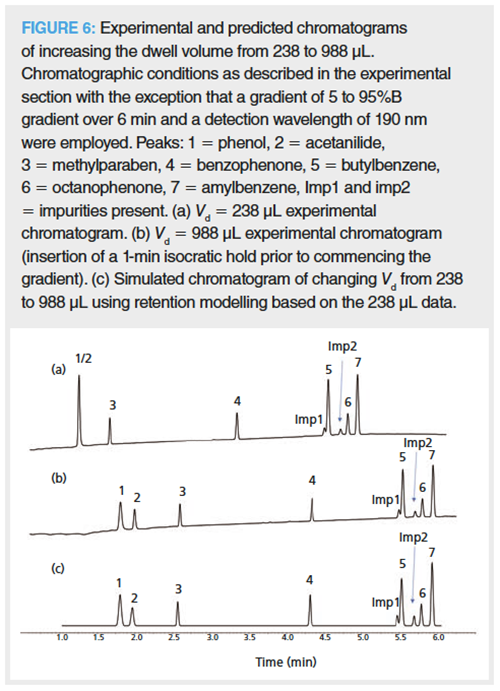
Once again, retention modelling was demonstrated to be able to predict the effect of increased dwell volumes on the chromatographic selectivity of the separation of these more hydrophobic analytes (Figure 6). For the more hydrophobic species, their chromatographic selectivity did not change as the dwell volume was increased because they are highly retained at the column inlet irrespective of the length of the initial isocratic conditions. In comparison, the two early eluting, more polar analytes, that is, phenol and acetanilide logD at pH 2.7 = 1.63 and 1.24, respectively, coeluted at low dwell volumes (Vd = 238 μL), while increasing the dwell volume (Vd = 988 μL) afforded their separation.
Conclusions
This work has highlighted the importance of dwell volumes in gradient reversed-phase chromatography. Despite the small disparities in dwell volumes between UHPLC (≈ 130–700 μL depending on the type of pump and mixer design or volume) and standard LC instruments (≈ 1000 μL), major selectivity differences can still be observed, including elution reversal, between LC systems possessing dissimilar dwell volumes. This has potential repercussions for practising chromatographers. This dwell volume theory should equally apply to other types of gradient LC including ion-exchange chromatography (IEC), hydrophilic interaction liquid chromatography (HILIC), and hydrophobic interaction chromatography (HIC). However, simple measures can be successfully applied to compensate for dwell volume variations. For example, a correctly translated and scaled methodology eliminates selectivity differences in gradient chromatography because of discrepancies in dwell volume (Vd/Vm) by the introduction of a delayed injection (Vd/Vm larger in new method) or an initial isocratic hold (Vd/Vm smaller in the new method). Dwell volumes can be readily determined as described in references 7 and 10. A description of the principles and potential pitfalls related to translations between differing LC formats can be found in a previous LCGC article (1). It is important that standard operating procedures (SOPs) for gradient chromatographic methods specify the dwell volume range that the method is valid for to facilitate the successful translation to LC systems with dissimilar dwell volumes. The robustness of gradient methodologies, with respect to dwell volume, can be conveniently assessed using retention modelling software.
Ideally, in gradient chromatography, the analytes must be highly retained at the column inlet under the initial gradient conditions otherwise they may start to migrate down the column in the dwell volume phase of the separation. If the analytes start to migrate, then different dwell volumes may lead to selectivity changes. However, if the analytes do not migrate during initial isocratic conditions of the analysis (that is, corresponding to the dwell volume) then the retention of all analytes will be affected to the same extent.
Acknowledgements
The authors would like to thank Santander Ltd and Shimadzu UK Ltd for funding a summer studentship for B. Vitiello and Novo Nordisk A/S (Denmark) for funding J. Field’s Ph.D. studies.
References
- P. Petersson, M.R. Euerby, and M. James, LCGC Europe 28(6), 310–320 (2015).
- J.W. Dolan, LCGC Europe 26(6), 330–336 (2013).
- L.R. Snyder and J.W. Dolan, High Performance Gradient Elution (John Wiley & Sons, Hoboken, New Jersey, USA, 2007).
- A.P. Schellinger and P.W. Carr, J. Chromatogr. A1077, 110–119 (2005).
- P. Jandera, J. Chromatogr. A1126, 195–218 (2006).
- http://www.acdlabs.com/resources/freeware/translator/ (accessed 16/1/2019)
- N. Lundell, J. Chromatogr. A639, 97–115 (1993).
- P. Jandera and J. Churácék, Gradient Elution in Column Liquid Chromatography (1st Ed. Theory and Practice, Elsevier, Amsterdam, The Netherlands, 1985).
- J.K. Field, M.R. Euerby, J. Lau, H. Thøgersen, and P. Petersson, J. Chromatogr. A (accepted for publication) (2019).
- P. Petersson, B.O. Boateng, J.K. Field, and M.R. Euerby, LCGC Europe31(3), 120–143 (2018).


Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.