Hot Topics Preview: A Universal Generic (U)HPLC Reversed-Phase Gradient Method for Quality Assessments of Multiple Small Molecule Drugs
LCGC Europe
Wouldn’t it be nice if a single generic high performance liquid chromatography (HPLC) method could be used for all small molecule drugs - not only for potency assays but also for ICH-compliant stability-indicating assays?
Wouldn’t it be nice if a single generic high performance liquid chromatography (HPLC) method could be used for all small molecule drugs - not only for potency assays but also for ICH-compliant stability-indicating assays?
The use of generic HPLC methods in pharmaceutical analysis is not new. For at least two decades, generic HPLC/UV/mass spectrometry (MS) methods using 1–2 min ballistic gradients have been used successfully for high-throughput screening of combinatorial libraries and new drug candidates (1). Similar generic methods are also used for in-process control (IPC) analysis to support organic synthesis or in quality control of raw materials (2).
The latest advances in ultrahigh-pressure liquid chromatography (UHPLC) systems and column technologies have enabled the development of a versatile, generic reversed-phase method(s) amenable to multiple small-molecule new chemical entities (NCEs) with an analysis time of 2 min for cleaning verification (3–4). The same universal methodology was found applicable for rapid characterization (3–6 min) of many drug substance samples with peak capacities over 200 and provides a useful starting point for the development of ICH-compliant stability-indicating assays of most small-molecule NCEs.
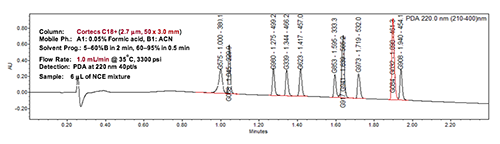
In this upcoming keynote presentation in HPLC 2019 in Milan, I will describe the development of this universal generic method(s) by selecting the best column technologies with optimized operating conditions for pharmaceutical analysis (superficially porous particles with bonding chemistries most compatible to highly basic compounds with simple mobile phases) (see Figure 1). The rationales used in the justification of the selection of various parameters will be disclosed.
Figure 1: The separation of 12 NCEs using the proposed universal generic gradient HPLC method.
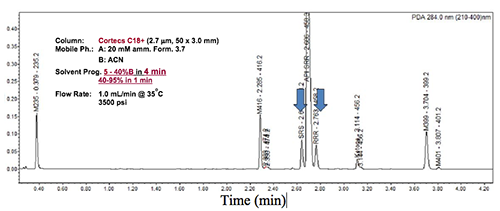
Case studies to illustrate the versatility of this generic method approach by simple adjustments of gradient range and time in the development of stability-indicating assays will be presented. These examples will include quality assessments of many NCEs including complex drug molecules with multiple chiral centres as shown in Figure 2.
Figure 2: The separation of a retention marker solution containing a complex NCE with three chiral centres spiked with expected impurities and degradation products (including two diastereomers). The 5-min method was obtained by adjustments of gradient parameters from the universal generic method, which took only one hour.
References
- M. Wong, B. Murphy, J.H. Pease, and M.W. Dong, LCGC North Am.33(6), 402–413 (2015).
- M.W. Dong, LCGC North Am.31(8), 612–621 (2013).
- M.W. Dong, E.X. Zhao, D.T. Yazzie, C.C. Gu, and J.D. Pellett, Amer. Pharm. Rev.15(6), 10–17 (2012).
- M.W. Dong, LCGC North Am.34(6), 408–419 (2016).

Michael W. Dong is a principal consultant in MWD Consulting focused on consulting and training services in HPLC and UHPLC, pharmaceutical analysis, and drug quality. He was formerly a Senior Scientist at Genentech, Research Fellow at Purdue Pharma, and Staff Scientist at Applied Biosystems / PerkinElmer. He holds a Ph.D. in analytical chemistry from the City University of New York, USA. He has 120+ publications including a bestselling book on chromatography (Modern HPLC for Practicing Scientists, Wiley). He is an editorial advisory board member of LCGC, American Pharmaceutical Review, and Chinese American Chromatography Association.

Silvia Radenkovic on Her Research and Passion for Scientific Collaboration
April 3rd 2025Radenkovic is a PhD candidate at KU Leuven and a member of FeMS. Her research focuses on inborn metabolic disorders (IMD), like congenital disorders of glycosylation (CDG), omics techniques such as tracer metabolomics, and different disease models.
Evaluating Natural Preservatives for Meat Products with Gas and Liquid Chromatography
April 1st 2025A study in Food Science & Nutrition evaluated the antioxidant and preservative effects of Epilobium angustifolium extract on beef burgers, finding that the extract influenced physicochemical properties, color stability, and lipid oxidation, with higher concentrations showing a prooxidant effect.