UHP-SEC Analysis of Biosimilars
The amounts of high- and low-molecular-weight impurities are critical quality attributes for a therapeutic protein. Size-exclusion chromatography (SEC), the standard technology for analyzing aggregation and fragmentation, was applied to elucidate the molecular similarity between an Adalimumab biosimilar antibody and the corresponding innovator product Humira.
The amounts of high- and low-molecular-weight impurities are critical quality attributes for a therapeutic protein. Size-exclusion chromatography (SEC), the standard technology for analyzing aggregation and fragmentation, was applied to elucidate the molecular similarity between an Adalimumab biosimilar antibody and the corresponding innovator product Humira®.
A biosimilar is a biological medicine highly similar to an approved biological medicine (originator) in terms of structure, biological activity and efficacy, safety, and immunogenicity profile. In order to demonstrate biosimilarity, the biosimilar manufacturer must generate an array of data comparing the proposed product to the approved reference product; a detailed analytical characterization and comparison of the products marks the first step in this process.
A series of analytical chromatographic techniques are used to characterize similarity. When it comes to the monitoring of aggregation and fragmentation of a therapeutic protein, SEC is the standard technology for the characterization of a biosimilar molecule versus the reference product. In this application, a TSKgel® UP-SW3000 UHP-SEC column was used to elucidate the molecular similarity between an Adalimumab biosimilar and the reference product Humira®.
UHPLC Conditions
Column: 30 cm × 4.6 mm, 2 µm TSKgel UP-SW3000
Mobile phase: 100 mmol/L KH2PO4/Na2HPO4, pH 6.7, 100 mmol/L Na2SO4, 0.05% NaN3
Gradient: Isocratic
Flow rate: 0.35 mL/min
Detection: UV @ 280 nm
Temperature: 25 °C
Injection vol.: 5 µL
Samples: Humira Innovator Reference Product (5 mg/mL); Humira Biosimilar (4 mg/mL)
Results
Humira and its biosimilar were then analyzed on a UHPLC instrument with UV detection using a TSKgel UP-SW3000, 2 µm SEC column in order to elucidate and compare the SEC profiles of the two molecules. Figure 1 shows that Humira and its biosimilar show slight variability in retention times. The zoomed-in profile provides a better look at the aggregates (HMW impurities and fragments) present in each sample.
Figure 1: UHPLC analysis of Humira (blue) and biosimilar (red) with TSKgel UP-SW3000.
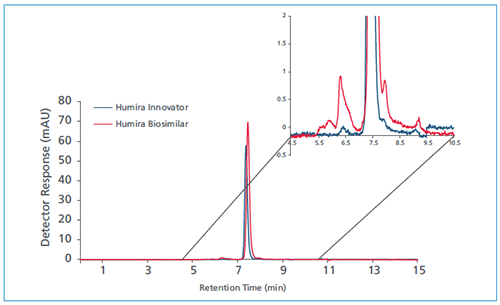
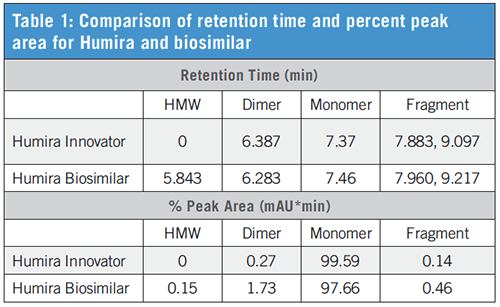
Table 1 outlines the retention time and relative percent peak area values that are shown in the chromatogram. The relative peak area calculation suggests a larger percentage of impurities, predominantly high-molecular-weight species (antibody aggregates), are present in the biosimilar compared to the innovator drug.
Analysis of six consecutive injections for Humira and its biosimilar. showed that results were highly reproducible.
Conclusion
UHP-SEC using a TSKgel UP-SW3000 column is a powerful tool to show the similarity between the innovator drug and the biosimilar with regard to the HMW and LMW impurities, as a function of hydrodynamic radii of the molecules. Reproducibility of consecutive injections yielded very low percent RSD when analyzing peak parameters such as retention time, peak area, peak asymmetry, and number of theoretical plates. Robustness and reproducibility are indispensable prerequisites for using SEC columns in quantitative analysis.
TSKgel and Tosoh Bioscience are registered trademarks of Tosoh Corporation
Humira is a registered trademark of AbbVie Inc.

Tosoh Bioscience GmbH
Im Leuschnerpark 4 64347 Griesheim, Darmstadt, Germany
Tel: +49 6155 7043700 Fax: +49 6155 8357900
E-mail: info.tbg@tosoh.com
Website: www.tosohbioscience.de

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








