Analysis of Phospholipid Classes by iHILIC®-Fusion
The Application Notebook
Phospholipids (PLs) are the major components of cellular membranes. They are important for the functionality of membrane proteins or serve as precursors for second messengers. Several studies reveal the role of PL alterations in various diseases such as cancer (1). Therefore, it is crucial to identify and quantify PLs in complex biological samples for lipidomic studies and clinical research.
Phospholipids (PLs) are the major components of cellular membranes. They are important for the functionality of membrane proteins or serve as precursors for second messengers. Several studies reveal the role of PL alterations in various diseases such as cancer (1). Therefore, it is crucial to identify and quantify PLs in complex biological samples for lipidomic studies and clinical research.
The PL classification is based on their different polar head groups. The diversity within each PL class is generated by combination of different fatty acids (FAs) with variation of chain length and the number of double bonds. Nowadays, tandem mass spectrometry (MS/MS) is the most important technique for the identification and quantification of PL species. By hyphenation with liquid chromatography (LC), LC–MS/MS provides prodigious power for the separation of isobaric or isomeric lipids. The amphiphilic properties of PLs mean that both reversed phase LC and hydrophilic interaction liquid chromatography (HILIC) are applicable for the separations. However, HILIC enables the separation of PL classes according to their polar head groups and minimizes ion suppression effects between PLs. The HILIC–MS/MS approach makes it possible to quantify lipid species based on similar retention times with internal standards of each lipid class (2) and also their characteristic fragments. Therefore, the assignment of lipid species to a specific lipid class is straightforward.
In this application, 10 PL classes are separated by HILIC using an iHILIC®-Fusion column. Detection was carried out by electrosprayâMS/MS. The applicability of this method for potential clinical application is demonstrated by the analysis of PL species in a lipid extract of a MCF-7 breast cancer cell culture (3).
Experimental
LC–MS system: Thermo Scientific Ultimate 3000 HPLC system coupled to a Q Exactive™ Plus Hybrid Quadrupole-Orbitrap™ mass spectrometer, which is equipped with a HESI II source and operated in negative ionization mode (ESI-).
Column: 150 × 2.1 mm, 3.5 µm 100 Å iHILIC®-Fusion (P/N 110.152.0310, HILICON).
Eluent: A) ammonium acetate solution (35 mM, pH 5.75) and acetonitrile (95:5, v/v); B) acetonitrile.
Gradient elution: 0–0.5 min, 97% B; 0.5–26.5 min, from 97% to 75% B; 27–33 min, 60% B; 35–45 min 97% B.
Flow rate: 0.3 mL/min
Column temperature: 40 °C
Injection volume: 10 µL
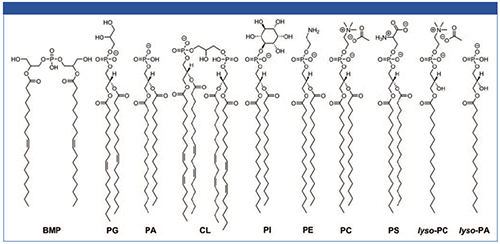
Phospholipid standards: Bis(monoacylglycero)phosphate (BMP 36:2), phosphatidylglycerol (PG 36:2), phosphatidylcholine (PC 32:0), phosphatidylethanolamine (PE 32:0), phosphatidylserine (PS 32:0), phosphatidylinositol (PI 32:0), lyso-phosphatidylcholine (LPC 16:0), phosphatidic acid (PA 32:0), lyso-phosphatidic acid (LPA 16:0), and cardiolipin (CL 72:8). The chemical structures are shown in Figure 1.
Figure 1: Chemical structures of phospholipids used in the study.
MCF-7 breast cancer cells: The extract of MCF-7 cell sample was provided by the working group of Dr. Cristina Cadenas (Leibniz Research Centre for Working Environment and Human Factors, Dortmund, Germany).
Results and Conclusion
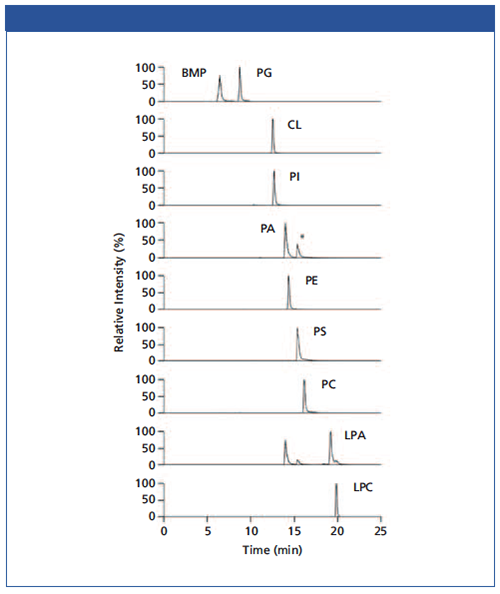
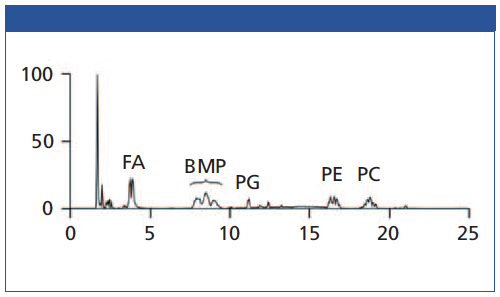
Figure 2 shows the separation of 10 PL standards according to their polar head groups and different chain lengths using an iHILIC®âFusion column. Importantly, the constitutional isomers BMP and PG were baseline separated. It was found that the other PL classes were also well separated. This is very important for the separation between PA and PS due to the potential in-source fragmentation of the PS head group leading to a mass-to-charge ratio (m/z) of the corresponding PA species. In short, the iHILIC®-Fusion provides good peak shapes and narrow peaks for all PL classes. The buffer concentration has turned out to be the most crucial factor for method development. It affected both separation efficiency and elution order. Furthermore, the acetate buffer also facilitated the detection of positively charged PC and lyso-PC species in the negative ionization mode as acetate adducts. All other investigated PL classes were detected as deprotonated molecules [M-H]−. Figure 3 shows the base peak chromatogram of a separation of MCF-7 cell extract. Besides our focused BMP and PG class, further PL classes such as PE or PC could also be well detected.
Figure 2: Separation of PL standards in ascending retention order with iHILIC-Fusion. *Double peak of PA is caused by in-source fragmentation of the PS head group resulting in corresponding PA species.
The combination of HILIC and tandem MS is a powerful tool for analysis of PL classes and PL species. The iHILIC®-Fusion provides an efficient separation of PL classes, which is mandatory for isomers such as PG and BMP (3). This facilitates a quantification approach based on single class-specific internal standards.
Figure 3: Chromatogram of PL class separation of MCF-7 cell extract with iHILIC-Fusion.
References
- C. Cadenas et al., Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1821, 1256–1268 (2012).
- E. Cífková et al., Anal. Chem. 84, 10064–10070 (2012).
- C. Vosse, C. Wienken, C. Cadenas, and H. Hayen, J. Chromatogr. A 1565, 105–113 (2018).

HILICON AB
Tvistevägen 48, SE-90736 Umeå, Sweden
Tel.: +46 (90) 193469
E-mail: info@hilicon.com
Website: www.hilicon.com










