Trends in Sample Preparation
Special Issues
A survey of LCGC readers on sample preparation techniques and methodology investigated trends in technologies being used, sample loads, sample sizes, automation, the use of SPE devices (cartridges, disks, plates, tips), SPE chemistries, selection criteria, and problems encountered.
The Significance of This Article-Then and Now
During my years with LCGC, I have collaborated with the magazine to prepare 16 surveys of readers, who, based on their profiles, are scientists working in the laboratory performing their daily tasks. I have always been amazed at the cooperation we receive from our loyal readers. In general, if a survey gets a 1-2% response rate, marketers consider the survey a success. Our reader surveys have generally gotten a response rate as high as 25%, even without an incentive to win a prize. The last reader survey I worked on is reproduced here, and it brought to light some trends in sample preparation use. Compared to our very first survey, discussed in the “Overview of Sample Preparation” article appearing in this supplement, this survey covers more than 39 techniques compared to only 19 in 1991. Compared to the last survey run in 2002, the number of laboratories automating their sample preparation increased modestly (29% compared to 26%) and the bulk (83%) consider their autosampler as their main sample preparation device even though most autosamplers merely inject a liquid sample. There was an uptick in the use of xyz liquid handling devices with 17% of the respondents using such a device. Still, 60% of respondents continue to feel that their biggest sample preparation headache is the time and labor intensity of their procedures. For future sample preparation endeavors, more automation, on-line solid-phase extraction (SPE) and 96-well plate versions, QuEChERS (quick, easy, cheap, effective, rugged, and safe), solid-phase microextraction (SPME), and miniaturized liquid extraction procedures were mentioned.
ABSTRACT
A survey of LCGC readers on sample preparation techniques and methodology was conducted in early January 2013. The results obtained were compared to a similar survey conducted a decade ago. Thus, the survey gave insights into trends in sample preparation over this time period. The survey investigated trends in technologies currently being used, sample loads, sample sizes, automation, the use of solid-phase extraction (SPE) devices (cartridges, disks, plates, tips), SPE chemistries, selection criteria, and problems encountered. Respondents were also asked about sample preparation technologies on the horizon; these new directions are summarized.
Despite the fact that many instrumental chromatographic techniques have matured and automation is commonplace, sample preparation is still considered slow, labor-intensive, and even a bottleneck in laboratory processes. Although certain high-throughput laboratories, particularly in the pharmaceutical industry and contract testing laboratories, use the latest automation equipment and process hundreds or sometimes thousands of samples a day, many laboratories are still using techniques based on age-old methodologies with some degree of miniaturization or low levels of automation taking place.
To keep readers and advertisers informed about marketplace trends, LCGC North America occasionally surveys readers on categories ranging from instrumentation preferences to high performance liquid chromatography (HPLC) and gas chromatography (GC) column information. In an effort to obtain a current profile of sample preparation practices, an internet-based survey was sent to a statistically representative group of LCGC readers at the beginning of 2013. The survey generated a total of 428 responses, which is statistically sufficient to look at some general observations and draw some broad-based conclusions on current sample preparation trends. The last survey was run in 2002 (1) with generally the same set of questions, which should allow me to look at trends in sample preparation technology in the last decade or so. This “Sample Prep Perspectives” installment summarizes the survey results and observes trends in sample preparation for chromatography.
Survey Audience and Sample Types
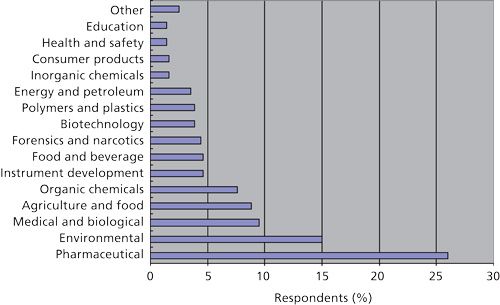
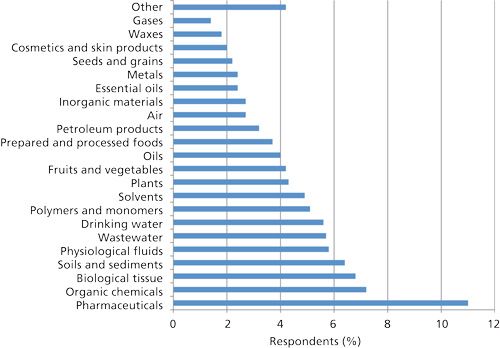
Figure 1 provides a breakdown of the fields of work of survey respondents and Figure 2 provides a Pareto chart of the typical sample types that they encounter. No doubt samples analyzed in your own laboratory fall into one of these categories. Most of the samples encountered require some pretreatment before injection into a chromatograph. In agreement with the 2002 survey (1) and with the respondent’s field of work (Figure 1), pharmaceuticals represent the largest single category followed by organic chemicals. The relative position of sample preparation of animal or biological tissues and physiological fluids (such as blood, urine, or cerebrospinal fluid) in which sample types fall into the current research work in genomics and proteomics has increased over the last survey while those reporting sample preparation of air and petroleum products has dropped. The relative increase in the position of sample preparation for fruits, vegetables, and plants has increased no doubt because of the increased scrutiny on our food supply. If one added up the total number of respondents analyzing every form of environmental sample (wastewater, soil, drinking water, sediment, or air) then this category would have stood out as the leading sample type. Obviously, when such a broad audience is surveyed, the physical state of samples should vary widely. When asked about the state of matter of the samples encountered, 95% of the respondents reported that they deal with liquids, 88% with solids, 48% with gases, and 53% with gels and semisolids, indicating that many of them deal with more than one type of sample.
Sample Preparation Techniques
Usually more than one sample pretreatment step is needed between sampling and the placement of the prepared sample into the chromatograph. Figure 3 depicts the 39 most cited sample preparation techniques used today in order of their popularity. The good news is that, relative to the 2002 survey (1), most of these techniques have seen an increased level of use, which indicates that there is a growing interest in the application of sample preparation. Marked increase in use in the last decade was noted for homogenization, blending, vortex mixing, and matrix solid-phase dispersion (MSPD). The first two techniques are very useful for preparing solid samples, and vortex mixing assists in quickly getting solid samples into solution or improving mixing. There have been a number of simple handheld devices that have come on the market for these techniques. MSPD is a useful technique for quickly extracting analytes from solid or semisolid samples, but it requires a bit of manual labor.
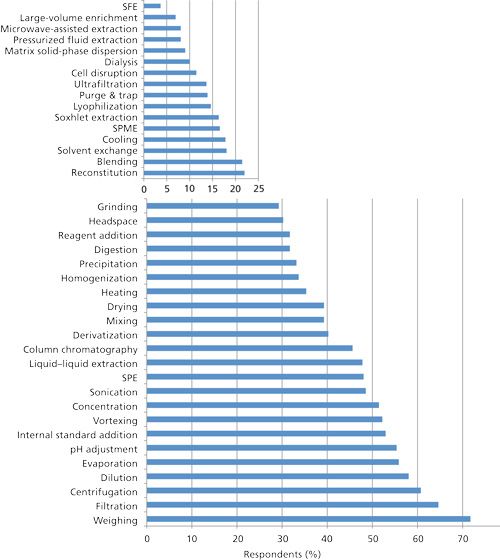
In Figure 3, some of the market-dependent techniques such as dialysis, cell disruption, ultrafiltration, and lyophilization are most often used for preparing biological samples. Other techniques such as large-volume trace enrichment and, to a lesser extent, solid-phase microextraction (SPME) are mainly applied in environmental analysis for trace analysis of organics in water. SPME does find applications in headspace analysis, but in this survey the headspace category was based on conventional gas-phase units. When the 2002 survey was conducted some newer extraction technologies had just appeared on the scene. In that survey, accelerated-solvent extraction (ASE)-also referred to as pressurized fluid extraction (PFE)-was used by 5.8% of the respondents and in the present survey 8% of the respondents are now using this technology. Quite surprisingly, microwave-assisted extraction was used by only 2.6% of the 2002 respondents while this year that number also jumped to 8%, the same as for ASE-PFE. Even though supercritical fluid extraction (SFE) was last on the popularity list, it still managed to eke out an increase in usage since 2002. All of these latter techniques are useful for the preparation of solid samples, usually under increased pressure and temperature. Microwave and ASE-PFE extractions are particularly fast relative to the classical solid extraction approaches with extraction times frequently less than 0.5 h. If the right types of samples are chosen, SFE can rival them in extraction speed and efficiency. The former techniques leave the sample in a diluted state but SFE can perform some level of concentration because the “solvent” supercritical carbon dioxide is quickly evaporated after the extraction.
When respondents were asked which techniques out of all the 39 techniques from Figure 3 they planned to use in the future, SPME, microwave-assisted extraction, ASE-PFE, solid-phase extraction (SPE), headspace sampling, and surprisingly even SFE all came out on top, indicating that these techniques may have a bright future.
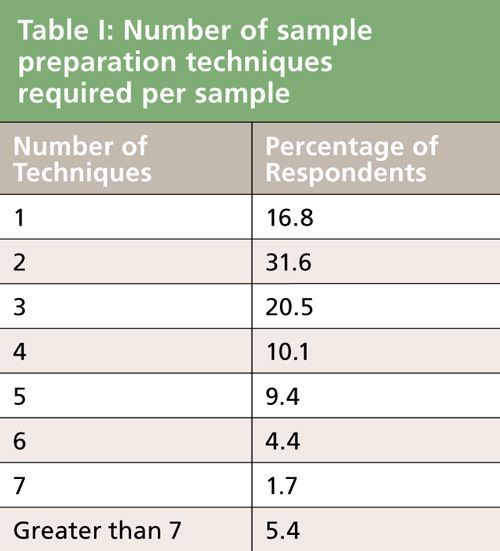
Very seldom is one lucky enough to be able to inject a sample with no pretreatment. Table I shows that 52% of the respondents use three or more techniques per sample. In fact, 5.4% of the respondents used seven or more individual techniques, which may indicate the complexity of their samples. The pattern looks very similar to the 2002 survey results (1). My estimation of the weighted average indicated that the average respondent used approximately three techniques per sample analyzed. For example, the use of liquid-liquid extraction (LLE) and evaporation to dryness of the collected analyte solution followed by reconstitution in a suitable solvent for injection into the chromatograph might be a typical three-step sequence for a liquid sample. In other cases, a single step, for example the “dilute and shoot” mode of a pharmaceutical liquid formulation might be all that is needed. The fewer sample preparation techniques before injection the better. A clear and optimum sample preparation strategy is needed to minimize the number of steps because each step represents additional time and a potential source of error. A trend brought about by the increased use of tandem mass spectrometry (MS–MS) with minimal sample preparation called “just enough” sample preparation (2) is something that we should explore in the next sample preparation survey.
Sample Loads
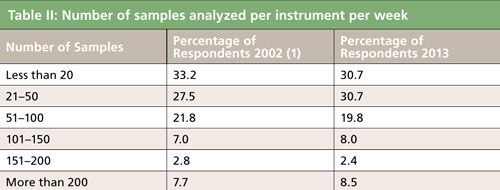
In the present survey, 48% of the respondents indicated that their sample loads will increase in the next two years while 4.6% said that loads would decrease; 47% indicated that the sample loads should remain the same. This year we queried the average number of samples analyzed per instrument per week and compared the data to the 2002 survey. Table II shows that comparison. Surprisingly, the data look fairly similar except that there was about a 10% increase in laboratories that push their instruments to analyze more than 200 samples per week. A slight drop off in laboratories that analyze fewer than 20 samples per week was also noted. High-throughput laboratories require some form of sample preparation automation unless their samples are very simple and do not require multiple steps to get the sample into their instruments.
Sample Characteristics
Initial Volume of Liquid Samples
Before injection into a chromatograph, the sample usually is in a liquid form. For liquid samples, the initial volume of sample can vary greatly. For example, trace organic contaminants that occur in environmental water may be in a large sample volume greater than a liter, but before analysis the organics must be trapped, concentrated, or enriched. Blood samples collected from neonates or tiny mammals may have volumes of less than 0.5 mL. One question in the survey asked about the initial volume of sample for those who had liquid samples. Overall, the current survey revealed that initial sample volumes encountered for liquid samples are decreasing. For example, in one of our first surveys in 1996 (3) 5.5% of the respondents had <1 mL of total sample and in 2002 (1) 18% of the respondents reported the same lower initial sample volume. In the present survey, 27% of the respondents had <1 mL to work with as their initial sample, continuing the trend in decreased sample volumes available. In fact, 14% had <0.5 mL of total sample.
On the larger end of the volume spectrum, respondents with >100 mL of sample stayed about the same in 1996 and 2001 (around 21%). This year’s survey showed that 7.9% of the respondents had sample volumes that were >100 mL, a big drop 10 years later. This observation may indicate that less sample is being collected and analyzed because the sensitivity of the instruments making the measurements have greatly increased in the last decade, thus requiring a smaller amount of sample to get an adequate analytical measurement.
Weight of Solid Samples
Solid samples must first be put into a liquid form, or soluble components of insoluble solids must be extracted. In our survey, we queried the initial sample mass in increments from 50 mg up to 50 g of total sample and within experimental error there was no particular trend in the mass available with the quantity of available sample being evenly spread among respondents.
Final Volume of Samples Before Injection
A benefit of most sample preparation techniques is the ability to concentrate the sample before injection. Respondents reported a range of typical sample volumes after concentration but before injection: <1 mL (38% vs. 25% in the 2002 survey), 1–2 mL (31% vs. 27% in 2002), 3–10 mL (14% vs. 29% in 2002), and >10 mL (17 vs. 19% in 2002). The trend seems that there may be better concentration tools nowadays than there were 10 years ago. A total of 69% of the respondents concentrate their samples to volumes of <2 mL. Sample volumes of <2 mL are suited to the standard 2-mL vials used in GC; HPLC instruments also use these vials but, in some cases, vials with volumes up to 5 mL are available. Microvials are also available for tiny volumes of sample (<100 µL) but we did not query this volume range. Of course, the actual volume injected into the chromatograph depends on the analyte concentration in the prepared samples. With modern injection techniques, large liquid volumes (>50 µL) can often be accommodated in both GC and HPLC.
Concentration
According to the present survey, 53% of the samples encountered have initial analyte concentrations of >1 ppm, exactly the same as in the 2002 survey (1) and 47% of the samples have concentrations of <1 ppm, again the same as in 2002. So, there was no apparent trend in sample concentrations becoming remarkably lower in the last 10 years. Concentration techniques such as SPE, LLE, and other extraction and evaporation techniques are still needed to analyze trace amounts of analytes. Optimized sample preparation techniques are critical for dilute concentrations. For these trace samples, the challenge of sample preparation is to ensure that sample loss does not occur by adsorption on the walls of the container, through evaporation, through oxidation, and the like. High enrichment factors are needed for the sample preparation methods. Techniques such as SPE or microextraction techniques can provide high enrichment factors. In addition, the use of sensitive detectors such as an electron-capture detector for GC and MS detectors in HPLC can be used to measure smaller amounts of analytes. For compounds that do not have strong chromophores or fluorophores in their molecular structure, derivatization will provide higher sensitivity. As indicated in Figure 3, 49% of the respondents indicated that they used derivatization at one time or another to improve detectability (or sometimes to improve separation characteristics).
Automation
The move to laboratory automation can occur for several reasons including
- higher sample loads,
- demand for higher productivity,
- the availability of fewer laboratory personnel or skilled workers,
- a need for better analytical precision,
- and the desire to avoid hazardous conditions such as toxic or highly radioactive samples.
With the increased sample loads noted at the high-volume portion of Table II (>200 samples per instrument per week), one would surmise that respondents would turn to automation to help increase sample throughput. The current survey showed that the number of respondents using automation is modestly up compared to 2002 (29% compared to 26% in 2002). Of those who don’t use automated methods, 55% explained that their sample throughput does not justify it and about half that number consider that the cost of automation isn’t justifiable. Considering that 61% of the survey respondents have, on the average, less than 8–10 samples/day per instrument (see Table II), this response is not surprising. About 27% of the respondents did not consider automation necessary, although 11% are reviewing possibilities for its use. However, of those only 4.2% of the respondents are reviewing the possibility of using automated sample preparation devices or plan to use such devices within the next 12 months.
When reviewing the “raw” data for respondent’s comments, some other concerns of turning to automation were cross contamination, too much variation in sample types or differing matrices that don’t lend themselves to automation, too big a variety of sample preparation procedures to automate, inability to find systems that provide the required accuracy and precision, samples sizes are too small, and assays are not designed for automation-all with some degree of credibility.
Automated Instrumentation for Sample Preparation
For those who indicated that they use automated equipment, 83% of them use autosamplers. Strictly speaking, autosamplers only allow direct injection of liquid samples but do not perform automated sample preparation functions. There are autosamplers on the market that do have limited sample preparation capability and 21% of the respondents indicated that they currently use such systems. Full laboratory robots can automate many manual tasks and can be found in many high throughput laboratories. However, in this survey the use of full laboratory robots has been flat since 2002. Apparently, the automation laboratories are finding more cost-effective productivity enhancement approaches than full laboratory robots. Marked growth was noted in using automated liquid-handling systems such as the xyz devices. In 1996, 8.4% of the respondents using automation employed xyz devices; in 2002 13.4% reported that they now use this type of automation and in the present survey this number has grown to 17%. Many of these devices that formerly only performed liquid-handling tasks have been adapted to handle other sample preparation techniques such as SPE (including cartridges, pipette tips, and well plates), solvent evaporation, and filtration. The other area of automation that has gained acceptance is the use of dedicated sample preparation workstations. In fact, nearly a third of the respondents indicated that they have at least one automated SPE system. Also, 23% of respondents have dedicated sample prep equipment such as evaporators and autodiluters that satisfy some of the drudgery of sample preparation tasks.
In the present survey, users were asked which sample preparation techniques from Figure 3 they are considering for automation. Here is the top 10 list in decreasing order of importance: headspace sampling, internal standard addition, flash (column) chromatography, SPE, evaporation, purge and trap, sample concentration, microwave-assisted extraction, dilution, and digestion.
Solid-Phase Extraction
SPE has been around for more than 30 years, since the introduction of the first packed cartridges in the late 1970s. The technique has seen a slow but steady growth over this time. In this survey, 48% of respondents use SPE, which is up significantly from 38% in the last survey (1). Nowadays, users have a choice of cartridges, disks, pipette tips, dispersive SPE, and 96-well SPE plates. SPE cartridges still maintain their lead (88% of respondents used them), but disks (37%) have seen decent growth since the 2002 survey in which 24% of respondents used them. The biggest advantage of the disk format is its ability to accommodate higher flow rates (and therefore shorter extraction times), which is most important in the trace enrichment of trace organics in water. The flow rate that can be passed through cartridges with their smaller cross-sectional areas is generally lower than flow rates used for the disks. The 96-well SPE flow-through plates were introduced in 1996 (3), and by 2002 14% of respondents to that survey had adopted them (1). Now, their use has nearly tripled and in this year’s survey 39% of respondents reported using them. The 96-well SPE plates have proven to be popular in pharmaceutical and contract laboratories that perform high-throughput drug assays. They are well suited for automation, and most liquid-handling systems have been adopted for their format. Aside from SPE use, 96-well plates are available to perform a multitude of tasking that used to take place in test tubes, for filtration and lipid removal from biological fluids. These plates are available with both silica- and polymer-based SPE packings. Usually silica-based SPE plates have up to 300 mg of packing per well while the polymer-based SPE plates have up to 50 mg per well. The polymer SPE plates are becoming quite popular because they have a higher capacity, are more forgiving when drying out, and allow generic SPE methods to be used.
The SPE pipette tips are also useful for automated systems because most liquid-handling devices are pipette-tip based. Over 50% of the respondents that use SPE have tried them for their cleanup work. A variety of SPE tips are available: packed, loose packing, and some with SPE sorbents attached to the walls so that flow is not impeded. Usually the SPE pipette tips are used for the isolation of small sample sizes, particularly for peptides. Dispersive SPE, in which bulk packing is used instead of a container, has become popular with the QuEChERS method (quick, easy, cheap, effective, rugged, and safe) where it is the second step in this growing sample preparation technique. In this survey we didn’t query the number of respondents currently using QuEChERS, but the fact that we received a number of write-in responses referring to the technique suggests that we should add it to the next survey.
In looking at the data a bit closer, 37% of the respondents who used SPE reported that they use cartridges 100% of the time, 11% use pipette tips 100% of the time, but only 3.6% for 96-well plates and 1.5% of disk users employ them 100% of the time. A total of 52% of the respondents use a mix of the SPE formats. Most likely, even those users who use plates probably do not use a plate if they only have a few samples to do or when they are developing methods. The plates are rather expensive to use if only a few of the wells are used and then the plate is discarded. SPE method development plates are available that have various chemistries or amounts of packing in the 96-wells. The idea is that the chemist develops the method on one of these plates and, after the method is developed, orders plates with the same amount and type of packing in each well.
Because of the rapid growth in polymeric SPE products over the past decade, we decided to survey polymeric and silica-based use separately. In terms of actual usage reported, 73% of the respondents who use SPE are using silica-based SPE phases while 62% are using polymeric phases. We didn’t fine tune the responses to query those using both but there is most likely some overlap in usage.
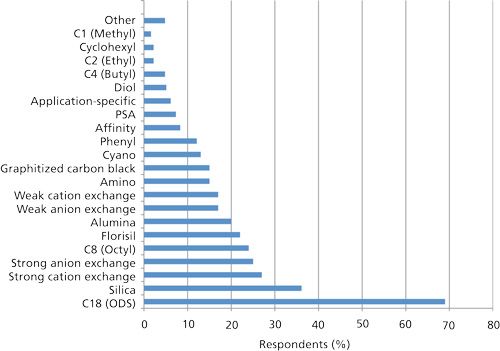
Figure 4 provides data about phase use from our current survey for silica-based SPE devices. Not surprisingly, overall the data somewhat resembles the pattern of HPLC modes usage data but the inclusion of the inorganic phases (for example, silica, Florisil, and alumina) and graphitized carbon black (GCB) that are used less in HPLC does negate the comparison. Reversed-phase chromatography is the most widely used separation mode in HPLC; likewise, the C18 phase is the top SPE phase with silica gel being a clear second. Silica gel is used more as an SPE cleanup phase than as a separation phase in HPLC and has always been popular as cleanup media in low-pressure column and flash chromatography. Silica gel-based SPE is performed in the normal-phase mode using organic solvents; samples are eluted with organic solvents and are concentrated to dryness with reconstitution in a solvent that is compatible with the mobile phases of reversed-phase chromatography. Florisil and alumina are also used for normal-phase SPE cleanup, particularly for pesticides and food samples for GC applications. Ion exchange has always been a popular technique for the rapid cleanup and concentration of ionic and ionizable compounds, and both cation- and anion-exchange SPE phases showed up very strong in the present survey as well.
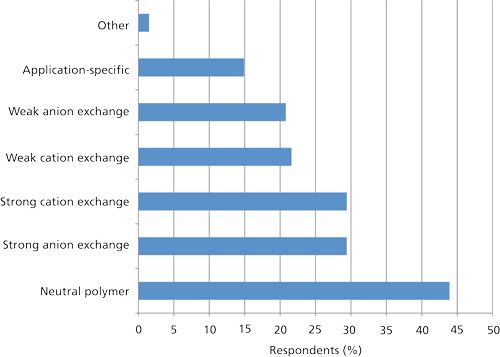
For the polymeric SPE phases, one can quickly see in Figure 5 that there are a lot fewer of them. Silica-based SPE has been around for decades while the polymer-based versions are newer. Nevertheless, polymeric SPE has really grown and the available phases seem to satisfy the current needs for sample cleanup. Just as for the silica-based SPE phases, the neutral polymeric phases were the number one type of phase in use. As mentioned earlier, generic methods are available, especially for their use in the cleanup of drugs and their metabolites in biological fluids. With silica-based phases, there is always some degree of method development involved.
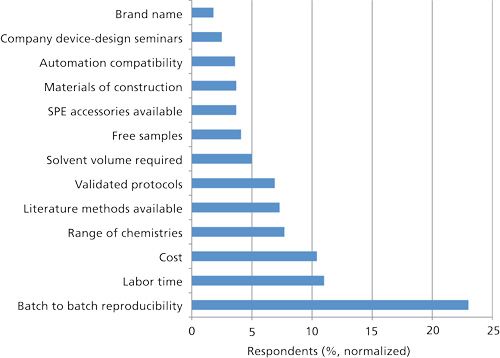
Chromatographers were asked to rate the relative importance (very important, somewhat important, important, and not important) of criteria used to select SPE devices (cartridges, disks, pipette tips, and plates) for sample isolation. To facilitate relative comparison, the data was normalized. Batch-to-batch reproducibility came out at the top of the list with 85% (23% on a relative basis) of the respondents rating this factor as very important. Obviously, nobody wants to develop an SPE method only to find out that the next box of cartridges or disks that they purchase cannot repeat their sample cleanup without redoing or tweaking the method. Reproducibility was followed by labor time and cost. Cost has always been lower in the list but perhaps the current economic climate has caused users to look more closely at their bottom line. Other very important selection factors included the range of chemistries available, validated protocols, and literature methods available-all of which can help to cut down on method development time.
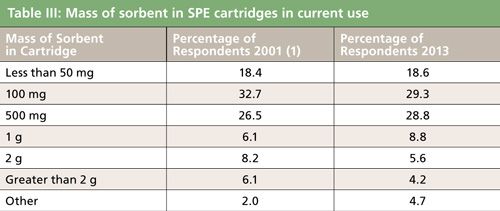
An important parameter in SPE is the mass of available stationary phase. The weight of the phase in the cartridge, disk, or SPE well plate determines the overall sample capacity, which is dictated by the amount of analyte as well as the level of matrix interferences. On the other hand, the smaller the SPE sorbent mass, the lower the amount of sample as well as the volumes of conditioning, washing, and elution solvent that are required. One of the biggest savings favoring smaller SPE sorbent mass is that the total cycle time is shortened, particularly the solvent evaporation step, which can sometimes limit sample throughput. Table III shows that in the present survey respondents reported that the amount of sorbent in the cartridges that they are now using has stayed roughly about the same as in 2002. The use of larger mass cartridges greater than 1 g/cartridge has decreased slightly and the use of cartridges with 100 mg/cartridge and lower has remained the same. For SPE disks, the top three sizes (diameters) were 47 mm, 20–30 mm, and 2–5 mm. In 96-well plates, the amount of sorbent is generally lower than 100 mg/well and the most frequently used masses were 1, 50, and 30 mg/well. For polymeric sorbents, the bed masses tend to be lower than the silica-based sorbent because their sample capacity is generally higher. Polymeric sorbents seem to be in favor in the 96-well plate configuration.
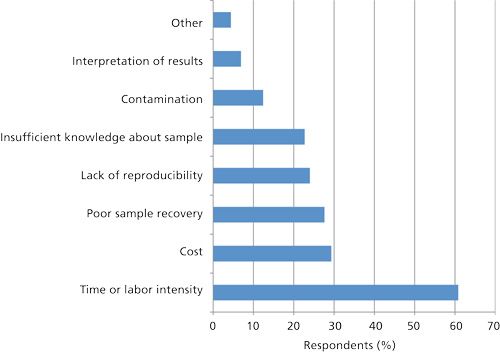
Top Problems Encountered in Sample Preparation
Respondents were asked to name the two top problems that they face in their general sample preparation and analysis. Figure 7 provides a Pareto chart of the top problems that users encounter. As might be expected, the time and labor involved in sample preparation tasks is still a big concern. Even though many new techniques and products are available to solve sample preparation challenges, users still are faced with the same old problem. Because the analytical steps are so much faster (with separations now being done in the 1–2 min time frame), the slowness of the sample preparation technique is exaggerated. Cost for sample preparation is a concern. With many sample preparation products now available in smaller sizes and with more sensitive instruments requiring fewer samples, one would think that the material costs should be decreasing. However, if processes aren’t automated then labor costs for manual sample preparation still comes into play. Sample recovery is probably a big concern with those doing trace analysis, but based on some of the observations in this survey, a lot of respondents aren’t routinely analyzing samples in the parts-per-billion range. Nevertheless, more inert sample preparation products would help in this regard. Certainly, with some of the new ultrainert GC systems and columns and HPLC or ultrahigh-pressure liquid chromatography (UHPLC) columns and tubing with PEEK lining and other deactivation approaches, the analysis part of the equation should be less of a contributor to poor sample recovery. Under the “Other” category, these were some of the more popular concerns: waste from sample preparation techniques, decreased column lifetimes, filtration difficulties, and optimization of sample preparation for multianalyte methods.
Emerging Sample Preparation Techniques
In this year’s survey, we asked each respondent to provide three sample preparation techniques that they felt could be commonplace or that they saw a need for in the next five years. Although not everybody answered this question, several respondents provided some interesting predictions or needs. Interestingly, more automation was a common theme, even though many of the respondents in this survey didn’t particularly warm up to current automation needs.
Increasing interest in SPE continues to be on the horizon with a number of respondents suggesting that on-line SPE could be more useful in solving their sample preparation problems. On-line SPE is really an automation capability because the technique could be controlled by valving operations in a typical HPLC system and programmed from the instrument controller. The on-line SPE columns can be used repeatedly, are in a closed system, and can be cleaned in situ. Thus, on-line SPE could cut costs, improve sample throughput, and provide better recovery than standard SPE. Increased use of SPME was also suggested both in a solution and headspace mode. Again, SPME is a path to automation because in its most popular form for GC, instruments are already capable of performing this technique unattended. Miniaturization of SPE even at the microfluidics or chip level was brought up as a future need.
Specific chemistry-based sample preparation like immunosorbents and molecularly imprinted polymers were again brought up as potentially improving sample preparation. These products have been around now for a decade and have be used in niche applications but are expensive and aren’t necessarily useable for repeated cycles.
Some respondents would like to see manufacturers provide autosamplers with more sample preparation features. Autosamplers are more compact than some of the benchtop liquid-handling devices and bring the sample proximity closer to the chromatograph.
Both microwave extraction and digestion were brought up as techniques that respondents are considering in the future. As noted in this present survey, microwave extraction has grown threefold since 2002 (1) and the use of microwave technology in synthesis and digestion has also been on the rise. One respondent suggested that microwaves might be applied to help with their distillation methods. PFE was also brought up as a technique being considered by some of the respondents. There are several ASE–PFE units now on the market some with simultaneous multisample capability.
QuEChERS was mentioned as a technique for the future. QuEChERS was originally suggested for the extraction of pesticides from fruits and vegetables, but more recently has expanded to other matrices, analytes, and toxins including diverse areas such as antibiotics in meats, drugs in whole blood, and anthocyanins in wine. Many suppliers are now providing ready-to-use kits for QuEChERS.
Some of the newer liquid extraction techniques such as dispersive liquid-liquid microextraction, membrane-based LLE, and supported liquid extraction (SLE) were mentioned as techniques that could become more mainstream in the future. Automated liquid-handling techniques such as autodilution, solvent exchange, automated micro LLE, dilute-and-shoot, on-line filtration, and evaporation or concentration were brought up as future needs.
Some more esoteric suggestions such as more applications of room-temperature ionic liquid (for a recent example see reference 4), on-line elution of dried blood spotting cards, precipitation (presumably protein precipitation of biofluids) directly into nuclear magnetic resonance tubes, and on-line polymerase chain reaction interfacing were brought up.
Conclusions
As was true in our last survey (1), sample preparation still gets attention in the chromatography laboratory. It is still considered a time and labor-intensive operation. Some definite trends in samples encountered and in their preparation were noted in this survey. Out of 39 sample preparation techniques surveyed, some of them extraction techniques for solid materials-microwave-assisted extraction, ASE, and matrix-assisted sorbent extraction-that were new a decade ago have shown moderate-to-strong growth. Sample loads have stayed about the same, but for liquid samples the initial volumes available decreased. Automation has shifted from full laboratory robots to modified xyz liquid-handling systems and dedicated sample preparation stations. Still a majority of respondents are not using automation and not even considering it in the near future. The introduction of 96-well SPE plates has accelerated sample throughput, especially in the pharmaceutical industry. Now, not only SPE plates but also filtration plates and SLE plates have made their appearance. Polymeric SPE sorbents have shown strong growth and are rivaling silica-based sorbents. Just as in HPLC, reversed-phase SPE dominates the phase technology for both silica- and polymer-based SPE products. Also, batch-to-batch reproducibility appears to be the most important parameter in the selection of an SPE device. The use of smaller sorbent masses in 96-well SPE plates seems to be growing.
Acknowledgment
I would like to thank those readers who took the time to respond to this rather lengthy survey. The information provided helps to keep all readers up on the latest technology in sample preparation.
References
- R.E. Majors, LCGC North Am. 20(12), 1098–1113 (2002).
- R.E. Majors, LCGC North Am. 30(12), 1024–1031 (2012).
- R.E. Majors, LCGC North Am. 14(9), 754–766 (1996).
- T.D. Ho, H. Yu, W.T.S. Cole, and J.L. Anderson, LCGC North Am. 31(2), 92–107 (2013).
How To Cite This Article
R.E. Majors, LCGC North Am. 31(3), 190–202 (2013).

Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)







