Overview of Sample Preparation
This first installment of the “Sample Prep Perspectives” column presents an overview of the primary manual and automated sample preparation techniques-methods that are much the same as those in use today.
The Significance of This Article-Then and Now
In 1990, I joined Hewlett-Packard as a manager in their Automated Chemical Systems group in Avondale, Pennsylvania, because I thought that sample preparation, the main thrust of the group, was an area that needed further attention. I had been writing in this magazine for eight years about liquid and gas chromatography columns, with an occasional deviation onto other topics, and I decided I should branch out and address sample preparation, given that I was now working in that area, which remained a hurdle in the analytical cycle. So we added “Sample Prep Perspectives” to my column-writing responsibilities. In January 1991, the first sample preparation article appeared, which is reproduced here.
For this special supplement, I chose this introductory article from nearly 25 years ago for a simple reason: Despite all of the work done and all the new sample preparation products and instruments produced, the majority of the information conveyed in this article remains the same today. Many laboratories still use age-old, time-consuming, manual, labor-intensive sample preparation methods that can still be a source of errors.
The full laboratory robots that were expected to become the panacea for automated sample preparation fell by the wayside. Managers had a hard time justifying the expense of purchasing a robot and then keeping a highly trained technical staff member to “baby sit” the instrument, watching for slipups. Some limited automation devices performing two or three procedures are now being used and some autosamplers have added additional capability to automate the last few steps in the sample preparation process. The miniaturization of some sample preparation techniques using 96-well plates has become more acceptable, but even techniques like the quick, easy, cheap, effective, rugged, and safe (QuEChERS) approach and solid-phase extraction (SPE) are still manual techniques in most laboratories.
ABSTRACT
Where is sample preparation headed? This first installment of “Sample Prep Perspectives” discussed the importance of sample preparation, manual and automated methods currently in use, and the trends that will shape sample processing in the future.
We can consider the development and application of an analytical method in terms of the sample-analysis flow diagram in Figure 1. Over the past several decades, considerable time has been devoted to improving analysis speed, resolution, and automation as well as to developing and improving instrumentation, data-handling, and report-generating software. In gas chromatography (GC), high-resolution capillary columns, sensitive rapid-response detectors, and linear, wide-dynamic-range analog-to-digital converters have improved the resolution, the sensitivity, and, especially, the speed of the technique. High-precision, pulseless pumps capable of reproducible isocratic and gradient solvent delivery, together with rapid, low-volume, leak-free injectors, microparticulate columns, and greatly improved detection capabilities, have made modern high performance liquid chromatography (HPLC) a widely used, reproducible, high-resolution, and fast analytical technique. Integrator- and PC-based chromatography data systems have improved the quality of the data and made data interpretation a relatively minor task. Laboratory information management systems allow the chemist or laboratory manager to monitor the analysis of samples after they have been placed in the instrument and subjected to multiple techniques.
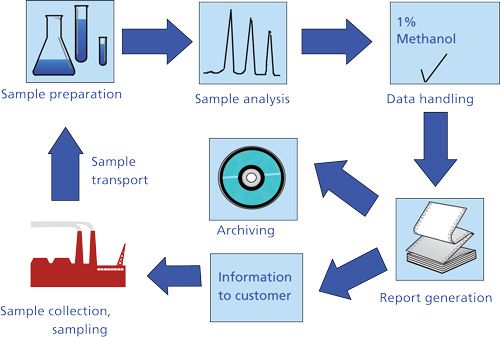
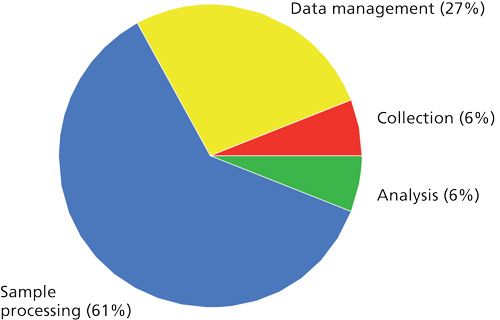
In contrast, sample processing, particularly its automation, has been neglected. Many analytical chemists use time-consuming manual methods that have been around for decades. Whereas an analytical procedure can require a few minutes--sometimes seconds--sample preparation time can be one or two orders of magnitude longer. The results of one survey (Figure 2) indicate that, for all respondents, two-thirds of the analysis time typically is spent on sample preparation, requiring more time than collection, analysis, and data management. Clearly, speeding up or automating the sample preparation step will reduce analysis time and improve sample throughput.
Currently, analytical chemists are very interested in new, faster sample preparation methods, especially automated methods. New products and instruments have been introduced onto the market. “Sample Prep Perspectives,” a new LCGC column, will report on the current state of sample preparation, mainly in conjunction with chromatographic applications, and will consider both manual and automated sample processing.
The Importance of Sample Preparation
Frequently, LCGC surveys its readers to better understand market trends and user needs. Last year, one survey included questions about sample preparation (1). Not surprisingly, when asked about the importance of sample preparation in their work, >90% of survey respondents indicated it was “very important” or “moderately important” (Figure 3). The introduction of a “clean” sample into a chromatograph makes separation easier and prolongs column life.

Another, often overlooked, aspect of sample preparation is its effect on error generation. Figure 4 provides survey data on error propagation for one method. Sample processing typically accounts for at least one-third of the error generated during the performance of an analytical method; operator-generated error is responsible for about 20%. Thus, improving and automating sample preparation can decrease the error of a typical analytical method by as much as 50%.
Because of the effect of sample preparation on both analysis time and error generation, automation of the procedure can minimize the time-consuming, labor-intensive, and error-prone aspects of a typical analytical method.

Goals and Objectives
The objective of sample preparation is to extract the analyte or analytes of interest from the sample matrix in the most concentrated form possible. In a chromatographic method, the sample should most often be injected into the chromatograph in a homogeneous solution. Because the analyte-removal technique can leave the analyte in a solvent that is undesirable or incompatible with the chromatography column or system, solvent sometimes must be exchanged. For example, when a buffered aqueous solution cannot be injected into a capillary GC column, solvent exchange is necessary. Similarly, in preparation for reversed-phase HPLC, the sample may be eluted from a solid-phase extraction (SPE) column using a strong solvent such as acetonitrile. Injection of a large volume of such a strong solvent into a reversed-phase column could cause the analyte to prematurely move down the column or spread over the column inlet, possibly resulting in a loss of resolution. In addition, concentration often is required when the analyte is present in a trace amount and detection is difficult.
Sample Types Encountered
In chromatography, the final sample should be in liquid form to be injected. However, the initial sample can be in various forms, as shown in Figure 5. Here, the “goos and creams” category does not refer to a new state of matter, but includes samples that are not clearly a solid or a liquid and whose room-temperature consistency can influence sample preparation. Of all sample types, solid samples are the most common. Newer techniques, such is supercritical-fluid extraction (SFE), are ideal for the rapid preparation of solid samples.

Most Frequently Used Techniques
Many sample preparation techniques are used in analytical laboratories. Some of the more popular ones are listed in Figure 6. Because this survey included all types of chromatography, it reflects overall use by both gas and liquid chromatographers. Obviously, weighing is the most popular technique because just about every quantitative analysis method requires sample weight measurement. Many of the methods shown in Figure 6 are candidates for automation. Many have been automated directly using laboratory robotics, whereas others use dedicated extraction or concentration instruments.

Trends
Chromatographers are looking for better sample preparation techniques to improve analysis speed and precision. They are looking for faster, more cost-effective procedures, and easy-to-use, convenient, and safer methods. For example, interest in SPE has grown not only because it provides better results (especially recovery and precision) than those produced by classical liquid-liquid extraction; SPE also requires less solvent and glassware, is less costly in labor and materials, speeds cleanup and methods development, and removes particulates without filtration.
Another trend is the increased use of smaller sample sizes. This trend is driven by the rapidly growing interest in biotechnology, in which analytical tests often are conducted on samples that are in limited supply. For example, protein-sequencing techniques using Edman degradation often require a small amount (sometimes in the micrograms-to-milligrams range) of purified polypeptide that may have been painstakingly isolated. Modern sample preparation procedures are expected to handle these small samples and provide good recovery without loss of biological activity.
Another trend in sample preparation is the growing need for automation. Automatic sample preparation can take several forms. First, one can emulate manual methods by duplicating the actions of the technician as he or she performs the analysis. This approach is often used in robotics: the robot duplicates the manual steps in the procedure, sometimes with the aid of specialized modules or “robot-compatible” peripherals that make access by the hand and arm more convenient. Robotics is an example of flexible automation whereby multiple functions can be performed using a single instrument (see Table I). Another type of automation is a device that performs part of the sample preparation process. Referred to as semiflexible automation, such devices can perform several tasks, such as SPE, filtration, and dilution. Some modern chromatography autosamplers fit into this category. These devices do not merely replace the technician’s manual procedure but can improve the sample preparation method. The third type of automation device performs one or two activities. For instance, so-called fixed or hard automation devices may perform only SPE.

Alternatively, an entirely new method or technology can be developed that greatly improves or replaces the manual method or technology. One example is the use of SFE in place of Soxhlet extraction to remove the additives in a polymer sample. Not only is SFE orders of magnitude faster (15-30 min) with equal or better recovery, but it can also be more easily automated and thus enhance sample throughput. Another example is the use of microwave digestion for the rapid dissolution of solid inorganic samples.
A case history of the analysis of diglycerides in flour (2) was studied to understand the advantages of adapting new technology to replace an existing manual method. The differences between the two approaches are evident in the “sample preparation wheels.” Figure 7a includes the sample preparation steps used in a typical manual method. Figure 7b presents the wheel for the analysis of diglycerides in flour, which involved multiple techniques including Soxhlet extraction, column chromatography, thin-layer chromatography, and pipetting. The published manual method for this analysis required 30 sample preparation steps before HPLC analysis of the di-glycerides. Each step is time-consuming, and subject to error because another sample manipulation is performed each time a transfer is made. Performing this method took 23 h. As the sample preparation wheel of Figure 7c shows, when SFE (solid-liquid extraction) was used, only five steps were required before HPLC analysis, which in this case took only two hours to complete-12 times less than the manual method.

Factors Influencing Purchase
One of the questions posed to readers in the survey (1) was, “What factors influence your purchase of sample preparation products?” Table II shows that high recovery (the amount of the desired analyte that survives the sample preparation process) and interference-free ingredients (the purity of the separated sample fraction) are the two most important factors in the chromatographer’s buying decision. The reduction of interferences is the SPE products, but the results could be applied to other sample preparation products as well.

The Future of Sample Preparation
Sample preparation will remain a fertile area of development in the future. New ways to increase the speed and efficiency of sample preparation processes will continue to be developed. Automation will save time and reduce error. Anticipated developments that will make sample preparation easier include new phases for SPE; improvements in hyphenated techniques such as zone electrophoresis-HPLC (ZEST) (3), micro-LC-GC (4), and SFE-SFC, -LC, or -GC; faster and more reliable robots; autosamplers that provide increased sample preparation capability; and a new generation of semiflexible automation devices.
References
1. LC-GC User Survey #6” (Aster Publishing Corporation, Metuchen, New Jersey, July 1985).
2. T.N. Tweeten, D.L. Wetzel, and O.K. Chung, J. Amer. Oil Chem. Soc. 58, 664 (1981).
3. A.J.J. Debets, K.-P. Hupe, U.A.Th. Brinkman, and W.Th. Kok, Chromatographia 29, 217 (1990).
4. H.J. Cortes, C.D. Pfeiffer , and B.E. Richter, J. High Resolut. Chromatogr. Chromatogr. Communications 8, 69 (1985).
How To Cite This Article
R.E. Majors, LCGC 9(1), 16-20 (1991).

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)







