Transient Protein Self-Association Determined by SEC–MALS
The Application Notebook
Wyatt Technology Corporation
Domain antibodies (dAbs) are the smallest functional binding units of antibodies, corresponding to the variable regions of either the heavy (VH) or light (VL) chains of human antibodies. Different dAbs can show different degrees of self-association, which drives the need for a cheap, quick — yet robust — technique to study self-association behaviour in drug discovery. This study outlines the use of SEC–MALS to determine self-association, and validates the work by comparison with analytical ultracentrifugation (AUC).
Methods
Shimazdu LC-20AD Prominence was used in series with miniDAWN TREOS and Optilab REX for this study. The columns used were TSK-GEL3000SWXL and Superdex S200.
dAb_1 was expressed, purified, and dialyzed into PBS, pH 7.4 and submitted to SEC–MALS and AUC. For MALS the sample was concentrated to 2.4 mg/mL, 7.0 mg/mL, 75 mg/mL, and 130 mg/mL and followed by appropriate dilution to obtain nine different concentrations that were run on SEC–MALS (Table 1).
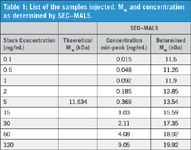
Table 1: List of the samples injected, Mw and concentration as determined by SECâMALS.
K2,1 (Propensity of dAb_1 to dimerize) was determined by plotting a graph of Mw versus log10 concentration obtained from SEC–MALS mid peak.

Figure 1: Overlay of UV, dRI, and MALS signal from SECâMALS for the sample with stock concentration 15 mg/mL.
The K2,1 values for dAb_1 from SEC–MALS was calculated as 300 μM compared to 391 μM from the AUC experiment.

Figure 2: Overlay of the five different sample results from SECâMALS highlighting the ’Determined Mw’. Samples with stock concentration 5 mg/mL, 15 mg/mL, 30 mg/mL, 60 mg/mL, and 120 mg/mL represent the smaller to bigger peak, respectively.
Conclusion
While AUC is the gold standard for the determination of self-association in solution, scientists are looking for alternatives as a result of AUC's high costs, low throughput, and expertise requirements. Here, SEC–MALS has shown excellent agreement with AUC results and can be used as a cheaper, easier, and quicker alternative.
This note was graciously submited by M. Burman and O. Schon, GlaxoSmithKline, 315 Cambridge Science Park, Cambridge CB4 0WG, United Kingdom.
Wyatt Technology Corporation
6300 Hollister Avenue, Santa Barbara, California 93117, USA
Tel: +1 (805) 681 9009 fax: +1 (805) 681 0123
Website: www.wyatt.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.








