Agilent Bio-Monolith Protein A Monitors Monoclonal Antibody Titer from Cell Cultures
The Application Notebook
Agilent Technologies
Analytical Protein A columns are used to determine the titer of monoclonal antibodies for the optimal time for harvest of the monoclonal antibody product. In this application note, Agilent Bio-Monolith Protein A columns are used to illustrate the quick capture of monoclonal antibody titer from cell supernatant.
Methods and Materials
Sodium phosphate monobasic monohydrate (Sigma p/n S3522), sodium phosphate dibasic anhydrous (Sigma p/n RES20908-A7), citric acid monohydrate (Sigma-Aldrich [p/n C1909]), and an Escherichia coli cell-lysis kit were purchased from Sigma-Aldrich Corp. (p/n CB0500). Humanized CHO-cell derived monoclonal antibody (IgG1) was purchased from Bio-Creative Labs.
Eluent A is used for equilibration, binding, and re-equilibration. This buffer contained 20 mM sodium phosphate buffer, pH 7.4. To make 1 L of 20 mM sodium phosphate buffer, pH 7.4, dissolve 3.1 g of NaH2PO4•H2O and 10.9 g of Na2HPO4 (anhydrous) in distilled H2O to make a volume of 1 L. This is 0.1 M (100 mM) sodium phosphate buffer with pH 7.4 at 25 °C. This buffer can be stored for up to 1 month at 4 °C. This stock solution is then diluted 1:5 with deionized water (take 200 mL of stock solution and add 800 mL deionized water) to obtain 20 mM sodium phosphate buffer, pH 7.4. Eluent B is used for protein elution and contains 0.1 M citric acid, pH 2.8. To make 1 L of citric acid buffer, weigh out 21 g of citric acid monohydrate, dissolve it in approximately 600 mL of water with gentle stirring, and adjust the pH with 1 M HCl until the pH is 2.8. Finally, dilute the solution with deionized water to the 1 L mark in a volumetric flask.
The manufacturer's recommended protocol was followed to obtain E. coli supernatant. The estimated concentration of protein was 40 mg/mL. The supernatant was then spiked with IgG1 as described; 40 mg/mL E. coli supernatant spiked with 2.5 mg/mL purified humanized IgG1. After mixing, the mixture was diluted further with mobile phase A (20 mM sodium phosphate buffer, pH 7.4) with a 1:1 ratio to a final concentration of 1.25 mg/mL of IgG1 and 20 mg/mL E. coli supernatant.
Results and Discussion
Quantitation for Titer of Monoclonal Antibody
Accurate quantitation of the titer and yield of monoclonal antibody helps to determine the amount of monoclonal antibody in the cell-culture lysate (supernatant) and indicate the harvest time. This is a very important step in manufacturing antibodies. To demonstrate the ability of the Bio-Monolith Protein A column in the quantitation of IgG1, different amounts (µg) of purified IgG1 were injected onto the column. Figure 1 shows the linearity areas (constructed by humanized IgG1 ranging from 0.625–5 µg) to the amounts of humanized IgG1 loaded on the column. This linear calibration curve is a standard curve. This correlation shows that the Bio-Monolith Protein A column can be used for quantitation of monoclonal antibodies in harvest cell-culture media with different concentration ranges. The column was also injected with 0.312 µg humanized IgG1 (data point not shown). The signal-to-noise (S/N) ratio here was higher than 1:1 ratio for the amount of 0.312 µg. The maximum loading capacity is approximately 400–500 µg IgG.

Figure 1: Agilent Bio-Monolith Protein A column quantitated monoclonal antibody.
In this example, Figure 2 shows data from 5–0.625 µg humanized IgG1 ranges. The column was also injected with 0.312 µg humanized IgG1 (data point not shown). The S/N ratio was higher than a 1:1 ratio in the amount of 0.312 µg. The maximum loading capacity is approximately 400–500 IgG.

Figure 2: The Agilent Bio-Monolith Protein A column quickly captures only IgG1 from a harvested cell culture spiked with IgG1. (a) E. coli cell lysate supernatant; (b) E. coli cell lysate supernatant mixed with IgG1. Injection: 2 µL (from 1.25 mg/mL IgG1 mixed with 20 mg/mL of E. coli supernatant).
Determination of IgG1 Titer from Harvested Cell Culture Supernatant
The specificity of the Bio-Monolith Protein A column is evident in Figure 2. When the supernatant (proteins from E. coli lysate) did not contain monoclonal antibody (IgG1), the chromatogram in Figure 2a shows no retention of any proteins. All proteins from the supernatant flowed through the column. The expansion of the baseline from Figure 2a provides further proof. Figure 2b shows the column captured and separated only monoclonal antibody (supernatant from E. coli spiked with IgG1). The amount of IgG1 was pre-determined and re-confirmed using the above standard curve (Figure 1) and was about 1.25 µg/µL. The Bio-Monolith Protein A column captured only the monoclonal antibody and eluted at approximately 1.4 min.

Figure 3: Binding of IgG1 with the column was evaluated at several flow rates. More sample was loaded for this study to easily observe changes in chromatogram and signal integration. Conditions same as figure one. Injection: 4 µL (1.25 mg/mL IgG1 mixed with 20 mg/mL E. coli proteins).
Effect of Flow Rate on IgG1 Binding, Peak Area, and Column Back Pressure
Three different flow rates, 1.0, 1.5 and 2.0 mL/min, were used to understand the effect of flow rate on IgG1 binding and column performance. There was very little effect of IgG1 binding on the column when the flow rate was increased (Table 1). The percentages of unbound proteins (E. coli proteins), relative area, and bound IgG1 relative area remained unchanged at different flow rates (Table 1).

Table 1: Flow rate versus peak relative area on unbound proteins and IgG1.
Typically, the Bio-Monolith Protein A column is recommended for operation at 1.0 mL/min. However, the column is rated to a maximum back pressure of 72 bar. Based on this particular instrument, at 1.0 mL/min, the column back pressure was 32 bar. When the flow rate was increased to 2.0 mL/min, the column back pressure increased to 68 bar. There was minimal effect on IgG1 binding onto the column at maximum back pressure, as indicated earlier. Therefore, it is possible to operate at a higher flow rate for faster analysis.
Salt Tolerance — Mixture of Cell-culture Supernatant and IgG1 with Different Salt Concentrations
In this experiment, supernatant mixed with IgG1 was dissolved with 0 mM to 200 mM NaCl to show the salt tolerance of the column. Results indicate that the Bio-Monolith Protein A column can tolerate samples with high salt concentration without deterioration of peak shapes. Calculations of peak areas from all three salt concentration show that any change was negligible (Table 2). More detail can be found in the full-length app note (publication 5991-2990EN).
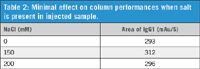
Table 2: Minimal effect on column performances when salt is present in injected sample.
The Influence of Different Elution Buffers
Data from Figure 4 show the influence of the elution buffers on the Bio-Monolith Protein A column. The data clearly indicate that IgG1 peak can be eluted using many different acidic buffers. Peak heights and retention times of IgG1 peak were observed. HCl, glycine, citric acid, and acetic acid with pH 2.8 and 100 mM were used. These elution buffers provide very comparable peak heights for IgG1, with variable retention times. Peak shapes and peak width are very similar in shape and width, except with 100 mM acetic acid. This elution buffer, when increased to 500 mM, gained enough strength to bring peak height and shape in line with other elution buffers. Therefore, depending on the acidic buffer, the concentration of the buffer needs to be determined.
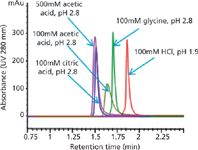
Figure 4: Influence of different elution buffers on IgG1 peak height and shape. Conditions same as Figure 1. Injection: 4 µL (1.25 mg/mL IgG1 mixed with 20 mg/mL E. coli proteins).
Reproducibility — Three Hundred Injections Before Clean-in-place
Data from Figure 4 and Table 3 show the results of 300 consecutive injections and are a clear indication of the column's reproducibility. The results indicate that the integrity of retention time, peak area, and peak width remained the same after 300 injections, without the column losing its binding and separating capacity for IgG1, and before clean-in-place.
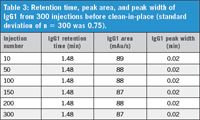
Table 3: Retention time, peak area, and peak width of IgG1 from 300 injections before clean-in-place (standard deviation of n = 300 was 0.75).
Conclusions
The Agilent Bio-Monolith Protein A column has a very high affinity for monoclonal antibodies. The column can capture and accurately quantitate monoclonal antibody exclusively from supernatant in less than 1.4 min to assess harvesting time. These columns can be used effectively to quantitate the amount of a monoclonal antibody with various flow rates without sacrificing data quality. The demonstrated flexibility of columns with different elution buffers as well as the tolerance for samples containing high salt concentrations allows experimental procedures to be easily designed.
To access the full appnote, with additional data on salt tolerance, regeneration, and clean-in-place, go to the Agilent library at www.agilent.com/chem/library and search for publication 5991-2990EN.

Agilent Technologies
2850 Centerville Road Wilmington, Delaware 19808, USA
Website: www.agilent.com/chem/advancebio

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.








