Extraction of Antiepileptic Drugs from Serum and Urine Using ISOLUTE® SLE+ Prior to LC–MS–MS Analysis
The Application Notebook
Biotage GB Ltd
Antiepileptic drugs (AEDs) are prescribed to suppress seizures in epilepsy patients. The ability to therapeutically monitor these drugs in patients is necessary for maintaining optimal medical care and managing any adverse effects of the drug. In this application note ISOLUTE SLE+ is demonstrated as an effective way to extract AEDs from serum and urine. The method was developed using a 96-well plate format to facilitate a high throughput workflow model.
Methodology
Format: ISOLUTE® SLE+ 400 μL 400 Supported Liquid Extraction plate, part number 820-0400-PO1
Sample pre-treatment: Pipette serum/urine (blank, calibrator, and patient [100 μL]) into a container. Add 50% aqueous formic acid (100 μL). Add up to 100 μL of internal standard.
Sample processing: Load up to 300 μL of pre-treated serum sample onto the ISOLUTE SLE+ 96-well plate. Apply a pulse of positive pressure or vacuum and allow samples to sit for 5 min.
Analyte elution: Elute analytes with methyl tert-butyl ether containing 1% (v/v) trifluoroacetic acid (conc) solution (2 × 700 μL). Allow sample to flow through by gravity and collect eluent. Apply positive pressure or vacuum as needed to facilitate a constant flow of approximately 1 mL/min (10–12 drops).
Post extraction: Evaporate to dryness (45 °C for 15 min) and reconstitute sample in mobile phase.
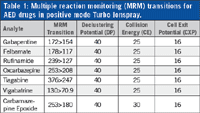
Table 1: Multiple reaction monitoring (MRM) transitions for AED drugs in positive mode Turbo Ionspray.
HPLC Conditions
Instrument: Agilent 1200 Liquid Handling System (Agilent Technologies, Berkshire, UK)
Column: Phenomenex Gemini C18, 150 mm × 4.6 mm (5 μm)
Mobile phase: Solvent A: 5 mM ammonium formate with 0.01% (v/v) formic acid; Solvent B: methanol:acetonitrile (50:50 m v/v)
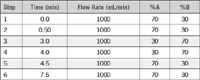
Gradient:
Mass Spectrometry Conditions
Instrument: Applied Biosystems/MDS Sciex 4000 Q-Trap triple quadrupole mass spectrometer (Applied Biosystems, Foster City, California, USA) equipped with a Turbo Ionspray® interface for mass analysis.
Ion source temperature: 700 °C
Results and Discussion
Use of an aqueous formic acid pre-treatment increases recovery of the zwitterionic gabapentine (Figure 1), compared with other pre-treatment strategies, but did not improve recovery of vigabatrine, because of its more polar nature. This results in a robust method giving high, reproducible analyte recoveries.
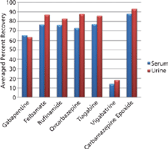
Figure 1: Plot of average recoveries for neutral and zwitterionic antiepileptic drugs at 20 ng/mL in serum and urine, pre-treated with 50% aqueous formic acid.
Biotage AB
Vimpelgatan 5, Uppsala, Sweden
Tel: +46 18 56 59 00 fax: +46 18 59 19 22
E-mail: info@biotage.com
Website: www.biotage.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.








