Tips & Tricks GPC/SEC: Finding the Right Standards
The Column
Polystyrenes (PS) are the most commonly used reference standards in gel permeation chromatography/size-exclusion chromatography (GPC/SEC) for nearly all organic GPC/SEC separations including high temperature GPC and pullulan or dextran for aqueous GPC/SEC. The majority of users rely on these standards for reproducible results. However, there is potential for improvements and this instalment of Tips & Tricks will discuss some general points that should be considered when determining calibration standards.
Daniela Held, Jasmin Preis, and Friedhelm Gores, PSS Polymer Standards Service GmbH, Mainz, Germany.
Polystyrenes (PS) are the most commonly used reference standards in gel permeation chromatography/size-exclusion chromatography (GPC/SEC) for nearly all organic GPC/SEC separations including high temperature GPC and pullulan or dextran for aqueous GPC/SEC. The majority of users rely on these standards for reproducible results. However, there is potential for improvements and this instalment of Tips & Tricks will discuss some general points that should be considered when determining calibration standards.
Gel permeation chromatography/size-exclusion chromatography (GPC/SEC) reference materials and standards are used for many different purposes. In addition to using them to validate a system, a detector, or to verify your own operational procedures, one of their main uses is to create a GPC/SEC calibration curve. Calibration curves provide extensive information about the chromatographic system and they allow us to:
- determine molar mass distributions and averages for unknown samples;
- measure pore sizes using inverse GPC/SEC for porous materials;
- quantify the resolution of GPC/SEC columns;
- identify the optimum separation range of GPC/SEC columns.
GPC/SEC reference materials are available for many different polymer types. Just as for GPC/SEC stationary phase materials, standards are very often classified either as aqueous standards for water-based GPC/SEC or as organic standards for GPC/SEC in organic solvents. There are a few standards that can be used for both applications.
Before deciding on a reference materials the following points should be considered.
What Reference Material is the Best Match for my Macromolecules?
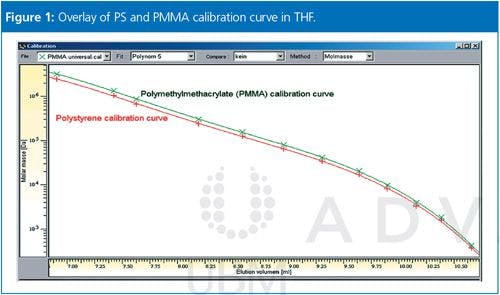
GPC/SEC is a relative method and the separation is based on the size (not the molar mass) of the molecule in solution. For example, a 100,000 Da polystyrene and a 100,000 Da polymethyl methacrylate (PMMA) differ in tetrahydrofuran (THF) by approximately 20% in size. Their calibration curves will not be the same, and therefore different results will be obtained for the molar mass distribution. Figure 1 shows an overlay of a PS and a PMMA calibration curve where it is clearly visible that the curves do not match.
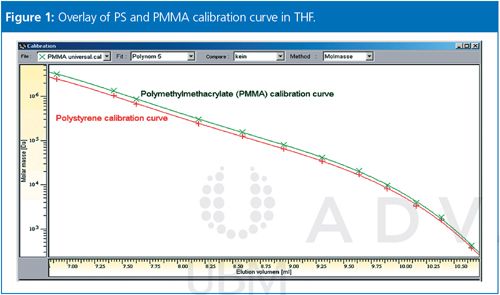
Please note that the shift between the curves is not just a linear shift with a constant factor. It is possible to use a mathematical procedure to transfer calibration curves into each other, but this process requires four constants in total, with the Mark-Houwink constants K and α for the two types of macromolecules involved.1
To obtain accurate molar masses it is best to use standards that are chemically and structurally alike to your samples. Therefore, a system that is used to characterize 1,4 polyisoprene, is best calibrated with narrowly distributed 1,4 polyisoprene standards. There are two advantages to this method. Firstly, all chromatographic problems can be easily identified when reference materials with known elution profiles are measured, and secondly accurate molar masses are obtained.
If no perfectly matching narrowly distributed reference materials are available several strategies can be applied to achieve reproducible or even accurate results:
Choose the standards that have the most similarity with your samples. Refer to the molar masses as “based on calibration with” as they will not be accurate but precise and reproducible.
If accurate molar masses are needed check for the availability of broad standards to perform a broad standard calibration.2 Producing your own (broad) standards is also possible. Another option is advanced detection. On-line light scattering3 is perfect for accurate molar masses of homo-polymers and proteins; on-line viscometry4 offers the chance to measure a universal calibration curve independent of the chemistry, structure, and composition of the calibrants.
Can the Standard be Used with my Mobile Phase/Stationary Phase?
An absolute prerequisite for using GPC/SEC is that the sample is completely soluble. This also holds true for the reference materials. Therefore, the reference materials are classified into materials for organic and for aqueous GPC/SEC. There are only a few examples where materials can be used in both solvent systems.
Polyethylene glycol (PEG) can be used in organic solvents such as THF for molar masses < 20,000 Da, but is also applied as a calibrant in aqueous GPC/SEC over a wider molar mass range. To overcome the high crystallinity, it is often required to heat the calibrant solution for a short time.
Also soluble in both solvent systems is poly(2-vinylpyridine). This material is often used for the calibration of GPC/SEC systems for polycation characterizations, but can also be used in other systems such as THF.
Polymethyl methacrylate (PMMA) is also widely used as a calibrant for organic GPC/SEC. Historically it has often been used in medium polar solvents such as dimethylacetamide (DMAc), dimethylformamide (DMF), or dimethyl sulphoxide (DMSO). The main reason for this was that previously only styrene-divinylbenzene polymeric phases were available; polystyrene could not be used because of interaction and retarded elution. Since the mid-1990s medium polar stationary phases have become commercially available allowing calibration with PS. However, in order to compare with older data, many results are still reported using PMMA as calibrants.
Similarly, PMMA is also used as a calibrant in hexafluoroisopropanol (HFIP) and is recommended for use in other solvents, such as THF or chloroform, especially if, for example, methacrylates are being investigated.
Pullulan is a reference standard used for aqueous GPC/SEC or for GPC/SEC in DMSO, for example when starches are characterized. It is a linear polysaccharide and therefore has some advantages over dextran. Dextran is normally branched and the branching increases with molar mass, that is, the structure changes. As the size of the higher molar mass dextrans only increase slightly with changing molar mass, it makes them less suitable for construction a calibration curve that makes full use of the complete separation range of the GPC/SEC columns.
Is Detection Possible with my GPC/SEC?
The standard detector for GPC/SEC is the refractive index detector (RI). Fortunately this detector is very universal and there are only a few isorefractive systems where this detector cannot be used in the applied solvent/sample combination. Therefore, nearly all reference materials can be used with RI detection. As an alternative to RI detection, evaporative light scattering detector (ELSD) can be used if there is sufficient difference in volatility between the standards and the eluent being used and if the eluent can be completely evaporated. It should also be noted that the response characteristics of an ELSD at low concentrations are slightly different from those of an RI.
If only an ultraviolet (UV) (diode array detector [DAD], photodiode array [PDA]) detector is available the choice of reference materials, especially for aqueous systems, is limited. Both dextran and pullulan, the two most common calibrants in aqueous GPC/SEC, are not UV-detectable in the vast majority of solvents applied. A UV-detectable alternative for neutral polymers and polyanions is poly(styrene sulphonate) sodium salt and poly(2-vinylpyridine) for polycations. Proteins can also be used.
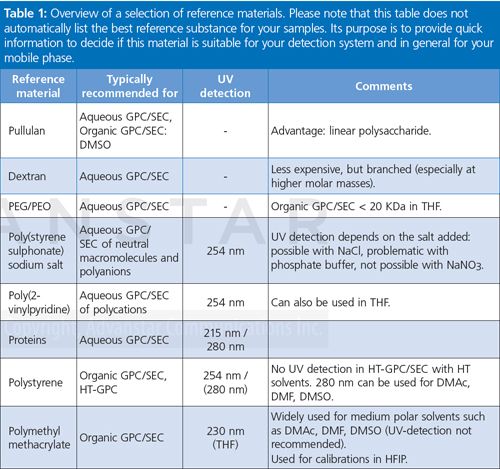
Table 1 shows a list of the most commonly applied reference materials with application recommendations. If UV detection is possible, an applicable UV wavelength is provided. Please note that this table does not automatically list the best reference substance for your samples, there might be better choices available. Its purpose is to provide quick information to decide if this material is suitable for your detection system and in general for your mobile phase.
Packaging of Calibration Standards
Normally polymeric reference materials are available as single standards with different molar masses. Kits are available to enable the fast and precise creation of calibration curves, which cover a dedicated molar mass range. The single standards selected for the kits ensure that the molar masses cover the complete range without gaps so that the data fitting process is more precise. The most convenient calibration kits consist of a mixture of different molar masses in the right concentration, which is premixed in autosampler vials. It is then only necessary to add the GPC/SEC mobile phase, wait a short time for full dissolution, and then inject directly from the vial.
Summary
GPC/SEC calibration standards are available with different chemistries to enable the determination of accurate molar masses.
Nearly all calibrants can be detected using RI detectors or ELSDs. Detection with only an UV (DAD/PDA) detector is limited, especially for the materials typically used in aqueous GPC/SEC.
Calibrants can be ordered as single molar masses or already assembled in kits or autosampler vial kits.
References
- D. Held, The Column4(6), 18-21 (2008).
- W. Radke and D. Held, The Column11(2), 10-15 (2015).
- D. Held and P. Kilz, The Column, 21-28 (2009).
- D. Held, The Column8(2), 12-16 (2012).
Daniela Held studied polymer chemistry in Mainz, Germany, and works in the PSS software and instrument department. She is also responsible for education and customer training.
Jasmin Preis studied polymer chemistry in Mainz, Germany, and works in the PSS production department. She is responsible for custom synthesis of specialty polymers and particles.
Friedhelm Gores studied polymer chemistry in Mainz, Germany, and works in the PSS contract analysis department. He is responsible for ambient and conventional GPC/SEC as well as hyphenation with light scattering and viscometry.

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.
What Goes in a CDS IT Service Level Agreement?
Published: April 7th 2025 | Updated: April 7th 2025Protecting your network chromatography data system (CDS) data is critical and a service level agreement (SLA) with your IT provider is vital. What should be included? Are SLAs for in-house IT and SaaS (software as a service) similar?
Advanced LC–MS Analysis for PFAS Analysis in Eggs
October 11th 2024The European Commission's regulation on maximum levels for certain contaminants in food highlights the need for precise and reliable methods to quantify per- and polyfluoroalkyl substances (PFAS) in various food matrices. This article discusses development and validation of a robust method for analyzing 21 PFAS compounds in chicken eggs using solid-phase extraction (SPE) and liquid chromatography–mass spectrometry (LC–MS).