Meeting the Challenges of Bioanalysis with Time-of-Flight High Resolution Mass Spectrometry (TOF-HRMS)
In an era where “more data in less time” is expected, bioanalytical scientists have had to become more resourceful. Consequently, bioanalytical laboratories are adopting and extending the use of high-resolution mass spectrometry (HRMS) and, in particular, time-of-flight high-resolution mass spectrometry (TOF-HRMS). One of the key benefits of TOF-HRMS is consistent resolution, sensitivity, and mass accuracy - even at high scan speeds with large molecules being analyzed.
Nigel P. Ewing, Waters Corporation, Milford, Massachusetts, USA.
Photo Credit: Digfoto/Getty Images

In an era where “more data in less time” is expected, bioanalytical scientists have had to become more resourceful. Consequently, bioanalytical laboratories are adopting and extending the use of high-resolution mass spectrometry (HRMS) and, in particular, time-of-flight high-resolution mass spectrometry (TOF-HRMS). One of the key benefits of TOF-HRMS is consistent resolution, sensitivity, and mass accuracy - even at high scan speeds with large molecules being analyzed.
Bioanalytical scientists have had to become more agile, resourceful, and flexible to obtain “more data and insight in less time”. As a result, many bioanalytical laboratories are adopting high-resolution mass spectrometry (HRMS) into their quantitative workflows.
HRMS technology comes in different forms, including electrostatic ion resonance mass spectrometry (ion trap MS); time of flight mass spectrometry (TOF-MS); hybrid quadrupole time-of-flight mass spectrometry (QTOF-MS), which involves tandem quadrupole isolation and/or fragmentation before transfer to TOF detection; and Fourier-transform ion cyclotron resonance (FTICR-) MS. The versatility of HRMS has been demonstrated in both quantitative and qualitative analyses and for small molecules, large molecules, and hybrid biotherapeutics.1,2 The recent evolution of more complex, targeted delivery methods such as antibody drug conjugates (ADCs) has resulted in HRMS becoming popular as the MS platform to complement traditional ligand binding assays.
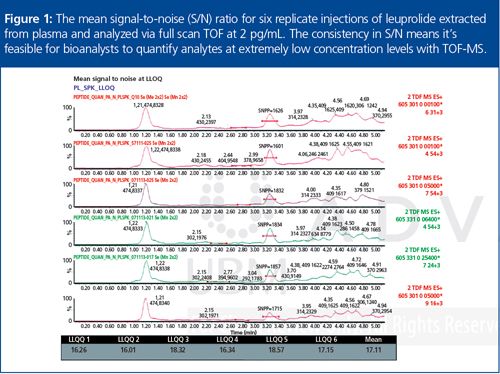
Despite the different capabilities of the respective HRMS platforms available, the unique advantages of TOF-HRMS will be the focus of this article. One of the key benefits of TOF-HRMS is consistent resolution, sensitivity, and mass accuracy - regardless of scan speed and molecule size. Recent modifications in both hardware and software have advanced analytical improvements in selectivity, sensitivity, and linear range. As a result, validated and transferrable methods using HRMS with both ultrahigh-pressure liquid chromatography (UHPLC) and microflow LC are being reported and one example employing leuprolide is shown in Figure 1.3,4,5 With this evolution in technology, the dossier of evidence for the acceptance and adoption of HRMS quantitation into regulatory environments is increasing.
A decade ago, tandem quadrupoles were the instrument of choice for LC-MS quantitation.3 Improvements in scan speed, sensitivity and selectivity, reduction in data file size, and instrument robustness have made TOF-HRMS a viable and complementary option for both qualitative and quantitative applications. For small molecule analysis, TOF-HRMS maintains its analytical performance, even with the narrow peaks obtained with fast UHPLC methods. Consequently, both full scan and multiple reaction monitoring (MRM) scan data can be collected without losing peak definition. In one example, quantitation of the parent molecule with complementary characterization of putative metabolites in a biotransformation workflow has been reported.2 This approach has also been implemented for residual analysis of pesticides and analysis of veterinary drugs in animal-based foods.5 In a recent review article, Rainville and colleagues described the robust use of a chip-based microfluidic separation device coupled with TOF-HRMS for characterization and bioanalysis of various biofluids with excellent inter-assay reproducibility. In the same report the beta-blocker, propranolol, showed clear improvement in the signal intensity and overall assay sensitivity when analyzed with the chip-based microfluidic platform compared with UHPLC.6
As the industry continues to shift from small molecule development and analysis to larger, more complex biomimetics and protein-based therapeutics, TOF-HRMS has proven to be a trusted companion along this journey. Although ligand binding assays still maintain a stronghold for quantitation of intact biotherapeutics like monoclonal antibodies (mAbs) and ADCs, the challenges associated with this approach (significant lead time for antibody generation, limited linear range of the assay, and the potential for cross-reactivity) are creating a space for ultrahigh-pressure liquid chromatography (UHPLC) or microflow LC-TOF-HRMS.1 These LC-MS platforms equip the analyst with the specificity, sensitivity, and flexibility to quantitate biomolecules. Although TOF-HRMS is well suited for the characterization of large, intact molecules, and desalted, deglycosylated proteins can be ionized and detected,7 quantitation is typically achieved through enzymatic digestion and analysis of a signature peptide with concomitant analysis of a stable isotope-labelled peptide analogue. For instance, Plumb et al. demonstrated the quantitation of a 70 kDa protein from plasma using a hybrid QTOF after enzymatic digestion. Although these authors observed good analytical merits (good linear regression and specificity), the ultimate desired outcome is reliable LC-MS quantification of intact proteins. However, as sample preparation, MS ionization, ion transfer, and detection techniques improve, it is anticipated that TOF-HRMS will lead the way to robust and routine quantitation of large intact biomolecules as a routine complement to ligand binding assays.
Although the regulatory agencies have widely accepted qualitative data from HRMS platforms for applications such as protein characterization, metabolite profiling, proteomics, and glycan analysis, evidence for the validation of quantitative data generated by TOF-HRMS is still growing, and the agencies have been slow to embrace this quantitative platform. Ramanathan notes that in regulated bioanalysis, the need for validation demands that the scope of the assay be carefully defined and that assay parameters stay validated for use over several years and even decades spanning the development life of the new chemical entity (NCE) from early clinical trials to late stage efforts and sometimes post-new drug application approval.3 As a result, it is more challenging in the regulated environment to establish methods on instrumentation that has been used historically for non-specific, non-targeted analysis because no precedent exists. This limitation is eroding. As investigators continue to show the utility of TOF-HRMS and transfer the methods to contract research organizations (CROs), it is anticipated that adoption and acceptance by the overseer of regulatory compliance in pharma and by extension, the regulatory agencies, will continue to increase.
In the last five years, a number of studies supporting the narrowing of differences in analytical sensitivity between tandem quadrupole and TOF-HRMS have been reported. In a study measuring prednisone and prednisolone in human plasma using full scan HRMS, the authors reported good accuracy (96-106%), good linear range (5-2500 ng/mL), and a lower limit of quantitation (LLOQ) of 5 ng/mL that matched the gold standard tandem quadrupole approach compared in the study.5 More recently, Evans and colleagues at GlaxoSmithKline (GSK) described the quantitation of a small molecule NCE using TOF-HRMS for improved selectivity, sensitivity, and successful transfer to a CRO. Dr. Evans’s team also developed a sensitive and selective TOF-HRMS method using chip-based microflow LC analysis for a large intact biomolecule with successful CRO transfer.8
The analytical challenges encountered by the bioanalytical scientist are growing and changing in complexity. In response to these obstacles, analysts have reached into their analytical tool box by using a new and improved HRMS resource. In the last 10 years, the scientific community has shown numerous examples where HRMS can match tandem quadrupoles for sensitivity. There is no compromise in speed of acquisition because the performance of TOF-HRMS is consistent at low and high data acquisition rates. TOF-HRMS maintains its resolution and is not affected by space charge effects associated with multiple charged biomolecules. As a result of these enhanced capabilities, HRMS in conjunction with UHPLC and microflow LC is knocking on the regulatory agencies’ doors and windows, serenading the investigators to pay attention and acknowledge its presence. In particular, as the applications and demand for characterization and analysis of biomolecules and drug conjugates continue to rise, TOF-HRMS will take wing and serve as a complementary analytical tool for ligand binding assays and enable the successful passage of these complicated biotherapies to the ultimate goal - new drug therapies for improved patient outcomes.
References
- R.S. Plumb, G. Fujimoto, J. Mather, W.B. Potts III, P.D. Rainville, N.J. Ellor, C. Evans, J.R. Kehler, and M.E. Szapacs, Bioanalysis4, 609-615 (2012).
- M-Q Huang, J. Lin, and N. Weng, Bioanalysis5, 1269-1276 (2013).
- D.M. Ramanathan, Bioanalysis5, 1141-1143 (2013).
- T.R. Croley, K.D. White, J. H. Callahan, and S.M. Musser, J. Am. Soc. Mass. Spectrom.23, 1569-1578 (2012).
- E.N. Fung, Y-Q Xi, A-F. Aubry, J. Zheng, and T.V. Olah, J. of Chromatogr. B 879, 2919-2927 (2011).
- P.D. Rainville, J.L. Langridge, M.D. Wrona, I.D. Wilson, and R.S. Plumb, Bioanalysis7, 1397-1411 (2015).
- T.R. Croley, K.D. White, J.H. Callahan, and S.M. Musser, J. Am. Soc. Mass Spectrom.23, 1569-1578 (2012).
- C. Evans, “The ever expanding role of HRMS in regulated bioanalysis applications to both small molecule and biopharmaceutical study support http://www.bioanalysis-zone.com/2015/07/28/the-ever-expanding-role-of-hrms-in-regulated-bioanalysis-application-to-both-small-molecule-and-biopharmaceutical-study-support/
Nigel P Ewing, PhD, is a Business Development Manager in Waters Corporation’s Pharmaceutical Business. His primary focus is on high-resolution mass spectrometry and its utility for small and large molecule quantitation.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Quantifying Terpenes in Hydrodistilled Cannabis sativa Essential Oil with GC-MS
April 21st 2025A recent study conducted at the University of Georgia, (Athens, Georgia) presented a validated method for quantifying 18 terpenes in Cannabis sativa essential oil, extracted via hydrodistillation. The method, utilizing gas chromatography–mass spectrometry (GC–MS) with selected ion monitoring (SIM), includes using internal standards (n-tridecane and octadecane) for accurate analysis, with key validation parameters—such as specificity, accuracy, precision, and detection limits—thoroughly assessed. LCGC International spoke to Noelle Joy of the University of Georgia, corresponding author of this paper discussing the method, about its creation and benefits it offers the analytical community.