Protein Aggregates and Gel Filtration Chromatography: Improving Quantitation and Throughput of a UHPLC Method
The Column
Gel filtration chromatography (GFC) is the most widely used method for quantitating protein aggregates in therapeutic drugs. It is a simple method, but prone to error as a result of poor method development and column selection. GFC columns tend to non-specifically adsorb large proteins and aggregates resulting in poor quantitation of “true” aggregate amount. Sample “priming” and mobile phase optimization can help reduce such irregularities. Simple method development rules using new column technologies are presented that demonstrate improved accuracy for these methods.
Michael McGinley, Zeshan Aqeel, and Brian Rivera, Phenomenex Inc, Torrance, California, USA.
Photo Credit: Alex Bramwell/Getty Images

Gel filtration chromatography (GFC) is the most widely used method for quantitating protein aggregates in therapeutic drugs. It is a simple method, but prone to error as a result of poor method development and column selection. GFC columns tend to non-specifically adsorb large proteins and aggregates resulting in poor quantitation of “true” aggregate amount. Sample “priming” and mobile phase optimization can help reduce such irregularities. Simple method development rules using new column technologies are presented that demonstrate improved accuracy for these methods.
In the world of protein therapeutic drugs there are several analytical assays that are routine for determining structure and function of proteins: peptide mapping, intact protein analysis by reversed phase, ion-exchange chromatography, and gel filtration chromatography (GFC). Although most of these analyses are used to look for structural changes in a protein, GFC is used to quantitate the aggregation state. This requires that the protein be in its native state, and therefore separation modes requiring denaturing conditions cannot be used. Aggregate analysis is necessary because of potential loss in specific activity of a protein as well as the strong possibility of immunogenicity for protein aggregates.
Unlike other chromatographic separation modes, GFC does not separate molecules by interactions with the stationary phase. Instead, molecules are primarily separated by differences in size and their ability to penetrate the pores of the stationary phase. Larger proteins can only partially permeate some of the pores of the fully porous media, which results in a reduced retention time; smaller proteins or small molecules can fully permeate all of the pore volume of a stationary phase, which results in longer retention times. Since protein aggregates are much larger (2× larger or more) than the protein therapeutic, they will elute earlier and relative peak heights or area can provide the analyst with an estimate of total aggregate present provided that all of the proteins remain soluble and do not bind to the GFC stationary phase. A key part of method development for GFC is based on maintaining solubility of protein and aggregate, as well as minimizing unwanted interactions between the stationary phase and analytes of interest.1
Secondary Interactions
The highest resolution gel filtration media on the market are typically fully porous silica media bonded with a highly polar ligand, usually a glycol- or diol-based chemistry. This “water-like” ligand minimizes any secondary interaction between the stationary phase and the protein analytes, thus leading to separation based primarily on the ability of the proteins to permeate the porous silica particle. Unfortunately, some secondary interactions can lead to anomalous retention characteristics for some proteins (especially very basic or hydrophobic proteins). Basic proteins can have electrostatic interactions with negatively charged silanol groups beneath the bonded layer of the silica particles. These interactions can lead to tailing peak shape, poor recovery, and increased retention of some basic proteins unless method development efforts are undertaken to reduce such interactions. Conversely, depending on the mobile phase, the glycol-/diol-based surface ligand can exhibit some hydrophobic behaviour. In the presence of a highly polar liquid phase (for example, a high salt buffer like 2X PBS [phosphate buffered saline]) these polar bonded phases can exhibit hydrophobic interactions between hydrophobic proteins and the stationary phase, similar to what is seen with hydrophobic interaction chromatography (HIC).
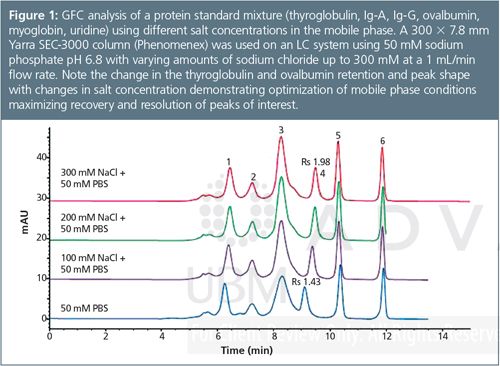
Therefore, every GFC method would benefit from optimization to properly minimize ionic and hydrophobic interactions. An excellent example of this behaviour is shown in Figure 1 where a mixture of standard proteins are run using different mobile phases of varying ionic strength. Depending on the recovery and resolution of proteins desired, one can optimize a separation while maintaining proteins in a native state. Some variant of a phosphate buffer either with or without supplemental salt is used to maximize the recovery and resolution of protein. An increasing concentration of phosphate buffer (50-150 millimolar, pH ~7) is preferred over using MS-friendly buffer systems, which tend to demonstrate low recovery and resolution of hydrophobic or ionic proteins; aggregates are especially negatively impacted. For very hydrophobic proteins (for example, antibody-drug conjugates [ADCs]), the addition of up to 15% isopropanol or acetonitrile to a phosphate buffer system has been reported by some to assist with the recovery of very hydrophobic proteins and is therefore an additional method development option to consider. Finally, some groups have shown that using arginine in the mobile phase can reduce secondary interactions. While such mobile phases minimize secondary interactions, the use of arginine in the mobile phase interferes with UV detection at lower wavelengths, thereby limiting detection to 280 nm wavelength.2
Stationary phase chemistry can also have some impact on the selection of the mobile phase. Some of the more classical GFC chemistries bonded to high silanol silica tend to benefit from the addition of excess salt (300 mM sodium chloride +) while newer column chemistries with low silanol activity tend to perform better with phosphate-only buffer systems. Of course, the pore size of the media (150Å vs. 250Å vs. 500Å) will have a major impact on the size range of the proteins being separated. It typically benefits a researcher to examine multiple pore sizes to get the optimal separation for a critical pair.
Flow Rate
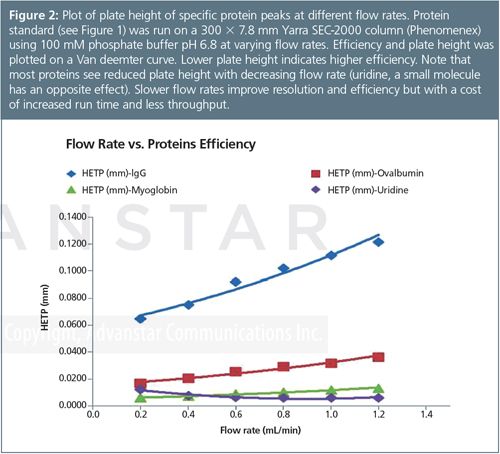
Flow rate is another overlooked optimization parameter to consider for GFC. Unlike other separation methods, GFC is not typically run at optimal flow rates. Large proteins diffuse much slower than small molecules and resolution of higher molecular weight species tend to improve as flow rate is decreased but at a cost of dramatically increased run time. Therefore, researchers trying to balance throughput in their laboratories are usually resigned to running their GFC separations at less than optimal conditions. A great example of the impact of flow rate on a separation is shown on Figure 2 where a standard is run at different flow rates using the same standard and mobile phase conditions. In the last few years new column chemistries have been introduced that use smaller particles (≤3 µm) to maintain higher resolution at accelerated flow rates. These columns offer an additional alternative when throughput is a consideration.
Prime Time
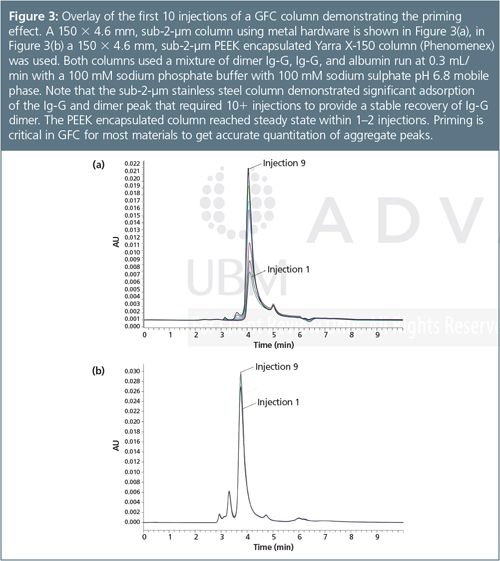
Minimizing secondary effects through good method development is crucial for GFC, but even with optimal conditions and an inert stationary phase some secondary interactions do occur. Residual silanol activity, interactions with the stainless steel of the high performance liquid chromatography (HPLC) column, and interactions with the frit material can all influence a GFC method. Often protein aggregate recovery is most impacted by non-specific adsorption, which can result in the aggregate present in a sample being underestimated. A common method to overcome such column limitations is the use of several “priming” injections of sample to “saturate” non-specific sites to get maximum recovery of any aggregate peak.3 An overlay of several injections in Figure 3(a) demonstrates the increasing recovery of protein with repetitive injections. While column chemistry has some impact on this phenomena, the recent introduction of a ultrahigh-pressure liquid chromatography (UHPLC) compatible PEEK hardware suggests that metal hardware of traditional columns also plays a role in non-specific adsorption and that the use of PEEK hardware (Figure 3[b]) might be a future solution for reducing the frequency of priming a GFC column before analysis.
Summary
Gel filtration is an easy separation mode to optimize to achieve good resolution and recovery of critical analytes of interest; however, many scientists just run PBS buffer and struggle through poor peak shape and reproducibility with questionable accuracy for quantitating aggregate protein. After screening columns that give the best separation with standard mobile phase (100 mM sodium phosphate pH 6.8), one can adjust salt concentration to optimize recovery and resolution of critical components. Alternate mobile phases or additives (arginine, 5-15% isopropanol, or even alternate salts) can also be considered if standard optimization is not efficient enough. Flow rate can also be adjusted based on resolution or throughput requirements. Recent introduction of 3-µm and sub-2-µm GFC columns allow for higher resolution in shorter analysis times compared to older GFC columns/chemistries. Finally, considering column priming and coming up with planned sequences of runs to assure a properly primed column are important for achieving accurate quantitation of aggregate in the sample of interest. Recent introductions of new materials and PEEK-encapsulated hardware can minimize or potentially eliminate the need for column priming. Using such method development strategies with a logical workflow can in most cases deliver an assay that gives accurate quantitation of the aggregation state of a therapeutic protein.
References
- M. McGinley. L. Mitre, and M. Klein, Intact Protein Analysis Method Strategies, Utilizing Approaches Based on Reversed-Phase and Gel Filtration Chromatography Bioprocessing Tutorial34(17), (2014).
- T. Arakawa, J.S. Philo, D. Ejima, K. Tsumoto, and F. Arisaka, Bioprocess International4(10), 42-49 (2006).
- P. Hong, S. Koza, and E. Bouvier, J. Liq. Chromatogr. Relat. Technol.35(20), 2923-2950 (2012).
Michael McGinley is the Core Product Manager at Phenomenex. He has a wide breadth of experience in chromatography and bioseparations and well over a hundred publications in the field.
Zeshan Aqeel is a Technical Specialist at Phenomenex with dozens of chromatography publications to his credit.
Brian Rivera is the Bioseparations Product Manager and manages Phenomenex’s portfolio of protein and peptide separation products.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Quantifying Terpenes in Hydrodistilled Cannabis sativa Essential Oil with GC-MS
April 21st 2025A recent study conducted at the University of Georgia, (Athens, Georgia) presented a validated method for quantifying 18 terpenes in Cannabis sativa essential oil, extracted via hydrodistillation. The method, utilizing gas chromatography–mass spectrometry (GC–MS) with selected ion monitoring (SIM), includes using internal standards (n-tridecane and octadecane) for accurate analysis, with key validation parameters—such as specificity, accuracy, precision, and detection limits—thoroughly assessed. LCGC International spoke to Noelle Joy of the University of Georgia, corresponding author of this paper discussing the method, about its creation and benefits it offers the analytical community.
Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.