Is There Really a Difference Between Flash and HPLC for LC Purification?
This note examines the benefits of using flash or HPLC for compound purification. Results show that flash is a viable alternative to HPLC.
L. Lloyd, S. Ball, and K. Mapp, Varian, Inc. — now a part of Agilent Technologies
This note examines the benefits of using flash or HPLC for compound purification. Results show that flash is a viable alternative to HPLC.
With the trend towards the use of smaller sub-2 µm particle materials for analytical HPLC to run the analysis faster, one would also expect a move to these materials for purification. While high efficiency separations performed at speed are utilized for purification, the capital cost of high-pressure equipment and columns makes the technique unsuitable for routine purification or when larger amounts of compound are required.
There are a number of factors that need to be considered before starting an LC purification of a target compound.
- Difficulty of the separation
- Required purity
- Required yield
- Amount of compound needed
- Purification throughput
- Available instrumentation
In all cases, the driver for the purification will be the economics of achieving the final compound or compounds at the required purity and with acceptable recovery.
A wide range of instruments, columns, and media are available for LC purification, ranging from flash to HPLC, and so we examined the advantages and disadvantages of using these techniques for compound purification.
Resolution Equation
Flash and HPLC are subsets of liquid chromatography and as such the same chromatographic theory will apply. The resolution equation (Equation 1) is applicable to both techniques. It contains three terms: efficiency, influenced by the particle size and shape of the media; selectivity, influenced by the total functionality of the media and eluents used for separation; and retention capacity, relating to the residence time of the solute in the column and influenced by the eluent composition.
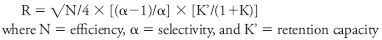
Equation 1: Resolution equation for liquid chromatography.
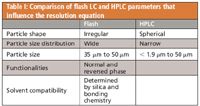
Table I: Comparison of flash LC and HPLC parameters that influence the resolution equation
Experimental Conditions
HPLC and flash columns are available with a wide range of media types. Two silica-based C18 materials were chosen in this study. The HPLC media was SepTech™ ST60 10-C18, a 60Å, 10-µm spherical silica particle, and the flash media was SuperFlash™ C18, a 60Å;, 50-µm irregular silica particle. Separation of a five-component test mix was used to assess the performance of the two materials (Figures 1A and 1B).

Figure 1: Selectivity comparison of SF10-5.5g C18 (A) and SepTech ST60 10-C18 (B), with 60:40 MeCN/H2O.
Column A: SuperFlash SF10-5.5 g C18
Column B: SepTech ST60 10-C18
Eluent: 60:40 Acetonitrile:water
Results
As would be expected, the peak widths were much broader with the SuperFlash column but the separations appeared comparable.
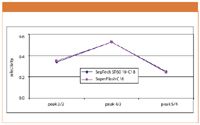
Figure 2: Comparison of SepTech ST60 10-C18 and SuperFlash C18 selectivity.
To determine differences in resolution between the SepTech and SuperFlash materials due to contributions from selectivity and retention capacity, these values were calculated for adjacent pairs of peaks. From the comparisons shown in Figures 2 and 3, it is clear that the two materials were very similar in their chromatographic performance. The difference in resolution of the sample components was due to the difference in efficiency, because of particle size and shape, and not to compound selectivity or retention capacity.
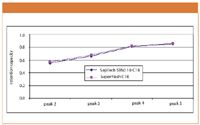
Figure 3: Comparison of SepTech ST60 10-C18 and SuperFlash C18 retention capacity.
Conclusions
Flash and HPLC are sub-sets of liquid chromatography and the resolution equation of LC is applicable to both. Efficiency, selectivity, and retention capacity components were evaluated for flash and HPLC media using SuperFlash C18 and SepTech ST60 10-C18 media, respectively. As expected from differences in particle shape, size, and size distribution, the biggest performance variations were seen in efficiency, otherwise the media were very similar in retention and selectivity. Therefore, where purifications do not require high efficiency, flash is a viable alternative to HPLC for compound purification.

Varian, Inc. — now a part of Agilent Technologies
2700 Mitchell Dr., Walnut Creek, CA 94598
tel. (925) 939-2400; fax (925) 945-2360
Website: www.varianinc.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














