The Role of Microextraction Techniques in Facilitating Gas Chromatography Separation of Complex Mixtures
This article addresses the latest developments in microextraction devices for gas chromatography (GC) analysis of complex samples with emphasis on biocompatible extraction phases. The role of microextraction in preventing “garbage in–garbage out” effects in one-dimensional (1D) and two-dimensional (2D) GC separation is described through various applications for food, environmental, and biological samples.
Since the first developments in the 1940s, gas chromatography (GC) has transformed separation science, providing robust and efficient separation platforms extensively used worldwide. Improvements, particularly in column geometries and stationary phase chemistry, have progressively facilitated the analysis of a variety of matrices. Furthermore, the development of multidimensional GC techniques has provided an ideal solution for the analysis of complex mixtures by expanding the chromatographic space available for separation and enabling peak focusing, especially via thermal modulation that permits analyte enrichment (1). Extraction and sample introduction methodologies for GC, however, did not follow the same evolution path, and for decades, these important aspects of the analytical workflow have been overlooked and underdeveloped. Headspace (HS) sampling, for example, has been the technique of choice for introducing volatiles into the gas chromatograph, preventing nonvolatile contaminants from entering the GC inlet. Often, however, the technique does not provide enough analyte preconcentration with low recoveries for semivolatiles, and requires a more sophisticated instrumental setup. Solvent injection in GC after liquid–liquid extraction, sorbent-based extraction techniques such as solid-phase extraction (SPE), or even sample dilute-and-shoot overcomes some of the shortcomings of HS sampling and provides a better option for analyzing semivolatiles. However, solvent injection is more prone to instrument contamination, especially when complex samples are analyzed. The invention of solid-phase microextraction (SPME) in the early 1990s provided a convenient solution for extraction and sample introduction into GC, considering its syringe-like geometry (more commonly known as fiber SPME) (2). SPME offered the ability for simultaneous extraction and preconcentration of the analytes of interest in a single and solvent-free step. The combination of HS sampling and SPME subsequently provided the perfect fit with one-dimensional (1D) and two-dimensional (2D) GC and expanded the analytical tools available for untargeted screening or quantitative target analysis. As a result, HS-SPME has been widely employed in the past three decades. This chapter of “microextraction history” is already well known and acknowledged by the scientific community. More recent developments in the configuration and chemistry of microextraction devices have significantly improved the role of this technique in facilitating GC separation, as described below.
Thin-Film SPME: An Alternative SPME Geometry for GC
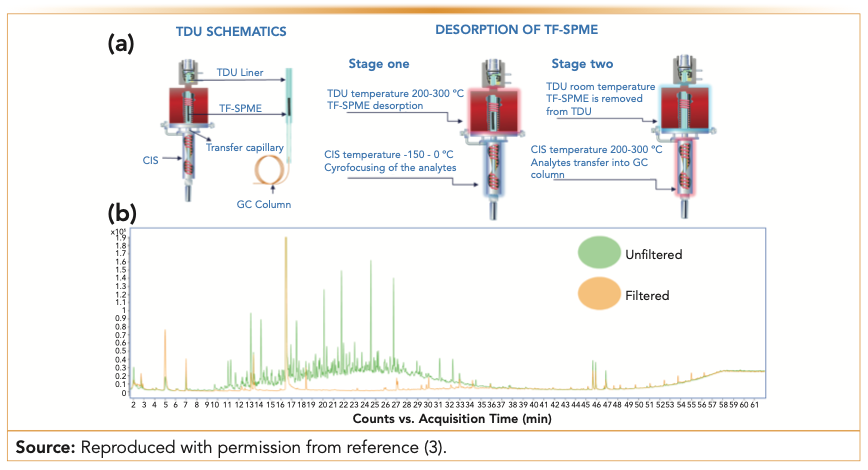
Thin-film SPME (TF-SPME) is a microextraction device that allows for depositing more extraction phases, thus having enhanced capacity compared to fiber SPME (3). The surface-area-to-volume ratio also allows for fast extraction kinetics, making TF-SPME devices ideal for ultratrace analysis. When hyphenated to GC, these devices must be desorbed in thermal desorption units (TDU) that are able to accommodate their size more conveniently compared to the conventional split–splitless injectors used to desorb SPME fibers. An advantage of using TDU units for the desorption of TF-SPME devices is the opportunity for cryofocusing the desorbed molecules when a programmable temperature vaporized injector is hyphenated. This dual-stage desorption and injection enables (Figure 1a) trapping and focusing of all the extracted analytes on the column head, greatly improving the efficiency of the separation and the sensitivity of the method. TF-SPME devices for GC analysis are available in various formats and chemistries (polydimethylsiloxane (PDMS), divinylbenzene/polydimethylsiloxane (DVB/PDMS), carboxen/polydimethylsiloxane (CAR/PDMS), and hydrophilic-lipophilic balanced/polydimethylsiloxane (HLB/PDMS)) for multiple headspace applications and direct immersion extraction of complex samples. Compared to conventional extraction techniques and fiber SPME, TF-SPME has shown enhanced extraction kinetics and has enabled the achievement of lower limits of quantitation for analysis of pollutants in environmental samples (4,5). The potential of this technique to facilitate GC separation of complex mixtures has been shown with its application to the characterization of produced water samples, a byproduct of fracking methods used for extraction of oil and natural gas (6). Produced water is an extremely complex matrix that includes dissolved solids, high salinity, and emulsion with petroleum products. The extraction of soluble organics via TF-SPME was performed via both headspace and direct immersion mode, guaranteeing comprehensive untargeted characterization (6). Moreover, it has been demonstrated that this approach can avoid the need for any sample pretreatment such as filtration (always needed for other extraction approaches to prevent contamination of the GC injector and column saturation by nonvolatile constituents), which enables recovery of a broader range of soluble organics than what can be achieved with other methods proposed in the literature (Figure 1b). This method, which is able to identify ~200 soluble organics present in produced water by 1D-GC, can be improved by using 2D-GC coupled to TF-SPME extraction to provide a better separation platform for the characterization of these complex samples. TF-SPME also has been hyphenated to portable GC–mass spectrometry (GC–MS) systems, providing a convenient solution for on-site sampling and guaranteeing enough preconcentration to allow detection of environmental pollutants at low parts per billion levels (7).
FIGURE 1: (a) Schematics of the two-stage thermal desorption unit for thin-film solid-phase microextraction (TF-SPME) (photo courtesy of Gerstel Inc.). CIS: cooled injection system; TDU: thermal desorption unit; GC: gas chromatography. Reproduced with permission from the literature (3). (b) Representative chromatograms obtained by TF-SPME-GC–MS analysis of untreated and filtered produced water samples.
Biocompatible Extraction Phases for Direct Immersion Extraction of Complex Samples
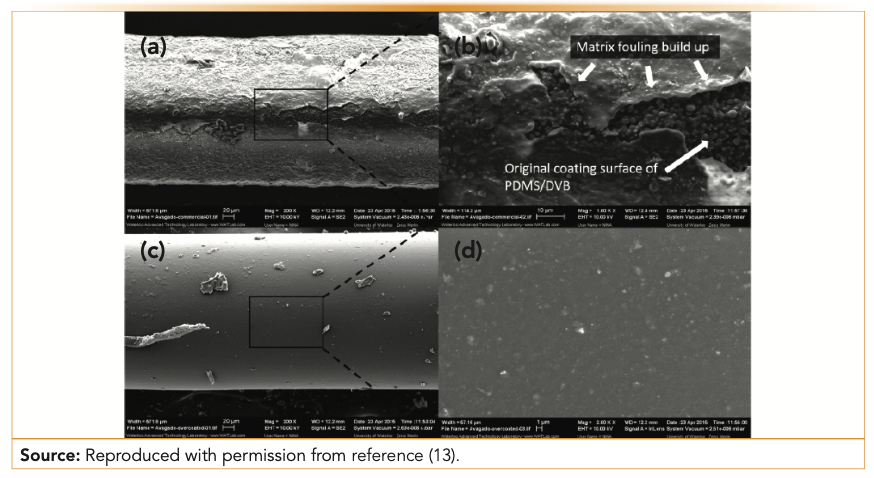
The development of biocompatible extraction phases for fiber SPME represented a turning point in the analysis of complex samples by SPME-GC. Although HS-SPME has been broadly applied over the years, limitations of the technique arose when analysis of organic molecules with a low Henry’s law constant (which do not partition efficiently into the sample headspace), was needed at parts per billion (ppb) or parts per trillion (ppt) levels. Although conventional extraction phases could be used for both HS or direct immersion SPME, the issues in the latter case consisted of the short lifetime of the extraction device because of fouling and introduction of contaminants or production of artifacts into the GC system. To overcome these limitations, a new extraction phase, PDMS/DVB/PDMS, was developed (8) and tested for a variety of food, environmental, and biological samples (9). These biocompatible extraction phases were found to be an excellent solution to prevent the production of artifacts (such as those generated in the GC injector at high temperatures) and thus, demonstrated their usefulness both for 1D- and 2D-GC separations (Figure 2) (10,11). For the analysis of fruits, Maillard reactions can take place in the GC injector when residues of carbohydrates and amines are present in extracts or on the surface of a nonbiocompatible SPME extraction phase. This phenomenon has been demonstrated for in vivo analysis of fruits, showing that even when 2D-GC is performed, the resolving power of the technique is still not enough to prevent these artifacts from masking the elution of minor metabolites peaks (12). The solution that biocompatible SPME phases provided was to prevent the attachment of carbohydrates on the extraction phase surface, thus eliminating the production of artifacts during their desorption in the GC injector and guaranteeing a “cleaner” chromatographic space where even trace metabolites could be detected. Another application, direct immersion extraction of a fatty sample such as an avocado, showed that biocompatible SPME devices are indispensable to eliminating the accumulation of nonvolatile fatty residues in the GC injector liner, which they are able to do because their inert surface prevents fouling of matrix constituents (Figure 3) (13), unlike with conventional SPME fibers.
FIGURE 2: Elution windows of GCxGC extracted ion chromatograms of furfural, a Maillard reaction byproduct, from in vivo metabolic profiles obtained by (a) DVB/CAR/PDMS and (b) PDMS-overcoated DVB/CAR/PDMS, a biocompatible extraction phase.
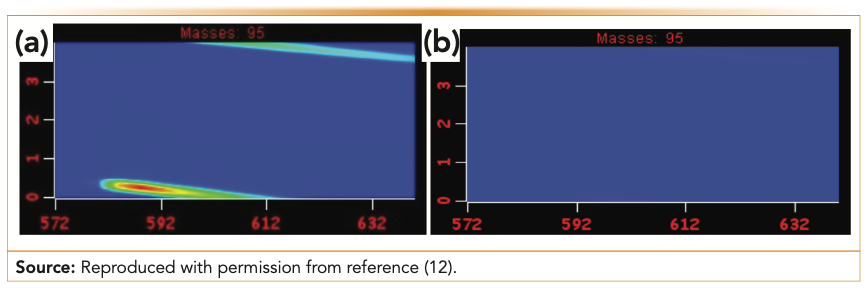
FIGURE 3: (a) Nonbiocompatible PDMS/DVB coating after 50 extractions in avocado samples (200x magnification); (b) magnification (1000x) of the PDMS/DVB coating surface; (c) biocompatible PDMS/DVB/PDMS coating after 100 consecutive extractions in avocado samples; and (d) magnification (2000x) of the PDMS/DVB/PDMS coating surface.
Conclusions
The role of microextraction in facilitating separation of complex samples via GC is obvious and has facilitated the development of important applications. Although technical developments in GC–such as sophisticated multidimensional GC systems and their hyphenation to high-resolution MS—that simplify the analysis of complex mixtures are also on the rise, their availability may not be widespread. For this reason, microextraction provides an affordable solution for preventing “garbage in–garbage out” effects in 1D- and 2D-GC separation. The ability of microextraction approaches to provide analyte preconcentration, interference removal, tuning of extraction coverage, an easy coupling to GC systems, and an opportunity for automation constitute the “most powerful analytical alliance” (14) with gas chromatography for targeted and untargeted analysis of complex samples.
References
(1) T Górecki, J Harynuk, and O Panić, J. Sep. Sci. 27, 359–379 (2004).
(2) C.L. Arthur and J. Pawliszyn, Anal. Chem. 62(19), 2145–2148 (1990). doi:10.1021/ac00218a019.
(3) R.V. Emmons, R. Tajali, and E. Gionfriddo, Separations 6(3), 39 (2019). doi:10.3390/separations6030039.
(4) H. Piri-Moghadam, E. Gionfriddo, A. Rodriguez-Lafuente, J.J. Grandy, H.L. Lord, T. Obal, and J. Pawliszyn, Anal. Chimica Acta 964, 74–84 (2017). doi:10.1016/j.aca.2017.02.014
(5) R.V. Emmons, A.M. Devasurendra, N.H. Godage, and E. Gionfriddo, LCGC North Am. 37(4S), 28 (2019).
(6) R.V. Emmons, T. Liden, K.A. Schug, and E. Gionfriddo, J. Sep. Sci. 43(9), 1915–1924 (2020). doi:10.1002/jssc.201901330.
(7) H. Piri-Moghadam, E. Gionfriddo, J.J. Grandy, M.N. Alam, and J. Pawliszyn, J. Chromatogr. A 1579, 20–30 (2018). doi:10.1016/j.chroma.2018.10.026.
(8) E.A. Souza-Silva and J. Pawliszyn, Anal. Chem. 84, 6933–6938 (2012). doi:dx.doi.org/10.1021/ac301305u.
(9) N.H. Godage and E. Gionfriddo, TrAC - Trends in Analytical Chemistry 111, 220–228 (2019). doi:10.1016/j.trac.2018.12.019.
(10) S. Risticevic, E.A. Souza-Silva, J.R. Deell, J. Cochran, and J. Pawliszyn, Anal. Chem. 88, 1266–12274 (2016). doi:10.1021/acs.analchem.5b03684.
(11) N. Reyes-Garcés, E. Gionfriddo, G.A. Gómez-Ríos, M.N. Alam, E. Boyaci, B. Bojko, V. Singh, J. Grandy, and J. Pawliszyn, Anal. Chem. 90(1), 302–360 (2018). doi:10.1021/acs.analchem.7b04502.
(12) S. Risticevic, E.A. Souza-Silva, J.R. DeEll, J. Cochran, and J. Pawliszyn, Anal. Chem. 88(2), 1266–1274 (2016). doi:10.1021/acs.analchem.5b03684.
(13) S. De Grazia, E. Gionfriddo, and J. Pawliszyn,. Talanta 167, 754–760 (2017).
(14) F.A. Franchina, D. Zanella, L.M. Dubois, and J.F. Focant, J. Sep. Sci. 44(1), 188–210 (2021). doi:10.1002/jssc.202000855.
Emanuela Gionfriddo is with the Department of Chemistry and Biochemistry, the Dr. Nina McClelland Laboratories for Water Chemistry and Environmental Analysis, and the School of Green Chemistry and Engineering at The University of Toledo in Toledo, Ohio. Direct correspondence to: Emanuela.Gionfriddo@UToledo.edu.









