Enhancing Flame Ionization Detector Capabilities with Post-Column Reaction
Groundbreaking performance improvements for the popular flame ionization detector (FID) were achieved by incorporating a post-column reaction with a three-dimensional (3D)-printed two-stage microreactor. Sensitivity improvements for compounds, such as carbon oxides, alcohols, aldehydes, ketones, chlorocarbons, and especially the new capability of the detector in achieving carbon universal response, are amongst the key highlights of the analytical strategy.
Gas chromatography (GC) is one of the most well-practiced chromatographic techniques in analytical chemistry (1–3). This ubiquitous technique delivers critical analytical solutions in many areas, from targeted compound analysis for indoor pollution monitoring to characterizing toxic chemicals in forensic investigations. Some of the reasons for the popularity of GC are that it is relatively inexpensive to implement, simple to operate, easy to maintain, and has a high degree of reliability. Despite more than 50 years of existence, the role of GC has not diminished or been replaced. Instead, the use of GC increases with continual developments in hardware and techniques to tackle a steady increase in sample complexity, catalyzed by societal transformations and initiatives such as increasing recycling and sustainability efforts.
The Flame Ionization Detector (FID)
Unlike other analytical chemistry disciplines, GC was blessed with many detector choices (4–6). Without any doubt, one of the most important innovations in GC was the invention of the flame ionization detector (FID) that was almost simultaneously and independently reported by I. McWilliam and associates (Australia) and J. Harley and associates (South Africa) in 1957 (7–10). Since its invention, the FID has become a choice detector in GC. Justifiable reasons for the FID to be in such a dominant position are as follows: It has a respectable detection limit around 0.5 pg C/sec, which allows the detector to meet many contemporary and demanding applications, and it is relatively inexpensive to construct, operate, and service, providing one can have access to fuel gases. Also, the detector can tolerate harsh chromatographic conditions of up to nearly 500 oC and highly corrosive gases, such as hydrogen chloride or sulfur hexafluoride. However, despite its wide-spectrum application and popularity, the FID is not a panacea for all chromatographic applications. The detector has some critical constraints. For example, compounds that can not generate CHO+ ions in a hydrogen flame, such as carbon monoxide, carbon dioxide, carbonyl sulfide, and formaldehyde, can hardly be detected. Also, the FID has a reduced response for some oxygenated or functionalized species. Another important issue critical for accurate quantification is that the FID has a highly variable response throughout many organic carbon-containing compounds and, in some instances, even within the same class of compounds like short-chain aldehydes or alcohols. Reference standards must be synthesized or procured to perform the analysis. Also, it is not possible to avoid handling compounds that are highly volatile, chemically unstable, or with an elevated level of toxicity.
Several attempts to improve the performance of the FID detector for targeted compounds have been reported in the literature. For instance, the measurement of trace carbon dioxide (CO2). This application attracts attention as CO2 is a major greenhouse gas that contributes to climate change. Catalytical reduction of CO2 to methane for detection by FID was first described by Porter and Volman (11), who demonstrated that carbon oxides could be converted to methane with an appropriate catalyst, such as nickel at an elevated temperature in a hydrogen atmosphere. Analytical devices based on this approach, commercially known as the “methanizer”, have been made available for many decades as a stand-alone or integrated chromatographic tool by various instrument suppliers and manufacturers (12). Although the device functions adequately in targeting carbon oxides in a relatively clean gas matrix, such as ambient air, it is susceptible to catalyst fouling because of poisoning by matrix constituents such as sulfur or halogenated compounds. Also, integrating the methanizer as an adjuvant device into the analytical system reduces the flexibility of the chromatograph while increasing the overall chromatographic void-volume of the system. The outcome of both effects is not desirable. A new reincarnation to this approach involves incorporating a more robust catalyst inside an FID jet (Jetanizer) to minimize void-volume and increase the system’s ease of service and platform flexibility (13–16). Although this approach improves chromatographic performance and robustness, the quest for carbon compound independent response capability by FID which is highly sought after by chromatography innovators, remained an elusive and moving target.
The Post-Column Two-Stage Reaction and Metal Three-Dimensional (3D)-Printing Technology
The post-column two-stage reaction has the potential of being a viable solution to carbon compound independent response capability for FID. In this reaction process, organic compounds are first oxidized to CO2 with a catalyst such as platinum and subsequently reduced to methane with another catalyst like nickel for detection as described in the literature (16–18). Although this strategy has merits, the extra column band broadening effect (σec) from having two packed bed reactors between the column outlet and the detector can be quite excessive and may negatively affect the overall chromatographic performance. A typical chromatography time domain of peak width in the range of 10 to 20 s was observed, making the technique not suitable for high-resolution gas chromatography.
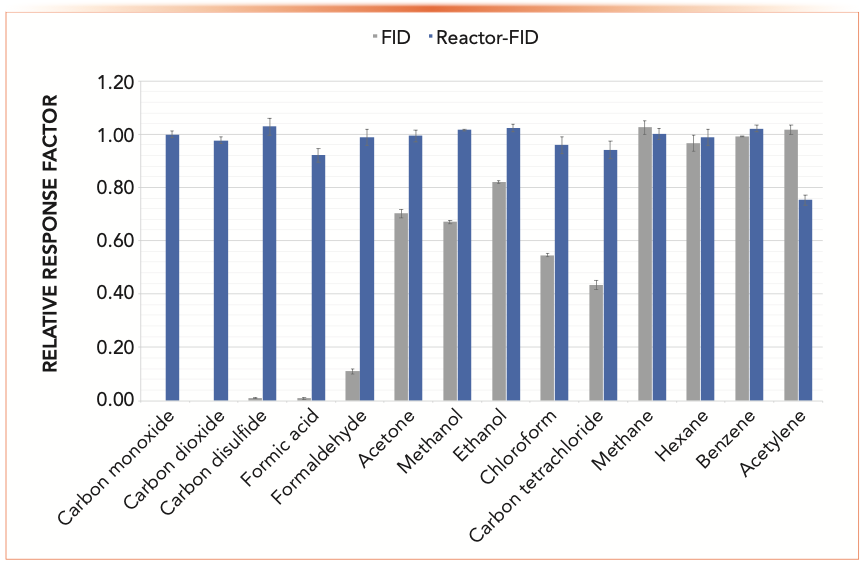
A novel approach to attain the same objective involves using metal 3D-printing technology for the fabrication of metal microstructure devices that are augmented with the appropriate catalysts in this microreactor embodiment (Polyarc). The assembly integrates two catalytic chambers in series where carbon-containing molecules are first converted to CO2 through a combustion process immediately followed by the methanation process (19,20). Figure 1 shows the relative responses to methane of model compounds from many classes of volatile and semivolatile organic compounds with the technique described. Several key enhancements are noted. First, the employment of the post-column reaction strategy expands the capability of the FID in that critical compounds of industrial significance with low or no response by FID alone, such as carbon monoxide, carbon dioxide, carbon disulfide, and formic acid, can now be detected with excellent sensitivity in the range of 0.5 pg to 1.0 pg C/sec with a contemporary FID. Second, as shown in Figure 1, sensitivity improvements were also observed for popular oxygenated compounds and chlorinated hydrocarbons, such as formaldehyde, methanol, carbon tetrachloride, and chloroform. In formaldehyde, an improvement of sensitivity of more than ten times compared to FID alone was observed. Finally, except for acetylene, where a reduced but reproducible response was noted, a universal carbon response was achieved. It was postulated that the reduced response of acetylene was caused by selective irreversible adsorption of the molecule by the catalysts. The universal carbon response resulted from the catalytic conversion of a carbon-containing molecule to methane, combined with the inherent wide linear range of the flame ionization detector for a compound such as methane affords compound independent calibration with a high degree of accuracy within ±5%. This new and convenient analytical capability has the potential of eliminating the need for an analyst to handle highly toxic compounds and thereby improving industrial hygiene and laboratory safety. The technique reduces or eliminates the need for multicomponent and multilevel calibration as well as enables the measurement of analytes with no known commercially available reference materials (19,20).
FIGURE 1: Universal carbon response and sensitivity enhancements for FID. Conditions are described in the literature (20).
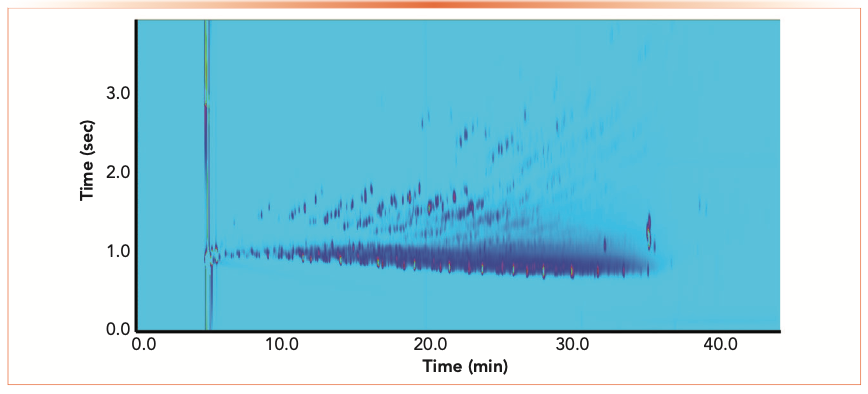
The suitability of the strategy with chromatographic techniques having the most stringent requirements on band broadening, such as high-resolution gas chromatography with columns having an internal diameter of less than 150 μm and GC×GC was demonstrated (19,20). Figure 2 shows a color plot of the separation of a Canadian winter diesel by GC×GC-FID with the microreactor incorporated. An average peak width at half-height (PWh) of 200 ms was achieved. As described earlier, with the added benefit of the analytical system’s universal carbon response, the sample constituents can be expediently quantified using a single compound standard.
FIGURE 2: Color plot of a commercial diesel fuel by GC×GC-microreactor-FID. Conditions are described in the literature (20).
Apart from the reduced response of acetylene, constraints associated with this technique include chromatographic peak tailing for silicon-containing compounds. This is because of the formation of active silica in the catalyst chambers. Although the peak tailing does not negatively impact equimolar response for carbon, the technique is contraindicated if trace analysis is required for said class compounds.
The strategy of employing post-column reaction to enhance FID performance is effective and reliable. The technique was successfully implemented for routine analysis in a quality control laboratory environment. Depending on the matrix involved, when properly maintained, the catalysts can last up to two years.
Overall, the approach is an impactful and relevant innovation in advancing GC.
Acknowledgments
Drs. Wayde Konze, Tonya Stockman, Linh Le, and Lotus Huang of the Dow Inc. are acknowledged for their support. Dr. Matthias Pursch, also of Dow, is acknowledged for his help in reviewing the manuscript. Dr. Andrew Jones of Activated Research Company is acknowledged for providing the prototype microreactor assembly for technology development and fruitful discussions.
This project is partially supported by the Dow 2019 and 2020 Analytical Science’s Capability Development Project Fund.
References
(1) C.W. Gehrke, R.L. Wixom, and E. Bayer, Eds., Chromatography: A Century of Discovery (Elsevier, Amsterdam, the Netherlands, 2001).
(2) K. Dettmer-Wilde and W. Engewald, Eds., Practical Gas Chromatography – A Comprehensive Reference (Springer, Heidelberg, Germany, 2014).
(3) O.D. Sparkman, Z. Penton, and F. Kitson, Gas Chromatography and Mass Spectrometry – A Practical Guide (Academic Press, Oxford, United Kingdom, 2011).
(4) H.H. Hill and D. McMinn, Eds., Detectors for Capillary Chromatography (John Wiley & Sons Inc., New York, New York, 1992).
(5) A.C. Nicholson, Eds., Detectors and Chromatography (Australian Scientific Industry Association, University of Melbourne, Australia, 1983).
(6) C. Poole, The Essence of Chromatography (Elsevier, Amsterdam, The Nether- lands, 2003).
(7) I.G. McWilliam and R.A. Dewar, Nature 181, 760 (1958).
(8) J. Harley, W. Nel, and V. Pretorius, Nature 181, 177–178 (1958).
(9) L.S. Ettre, LCGC Europe 15, 364–373 (2002).
(10) V. Pretorius, in 75 years of Chromatography – A Historical Dialogue, L.S. Ettre and A. Zlatkis, Eds. (Elsevier, Amsterdam, The Netherlands, 1979), pp. 333–338.
(11) K. Porter and D. Volman, Anal. Chem. 34, 748–749 (1962).
(12) C. Wang, Agilent Application note 5990–5129EN (2010).
(13) J. Luong, Y. Hua, R. Gras, and M. Hawryluk, Anal. Methods 10, 1275–1279 (2018).
(14) R. Gras, Y. Hua, J. Luong, P. Qiao, X.G. Yang, and P. Yang, J. Sep. Sci. 17, 2826– 2834 (2019).
(15) J. Luong, X. Yang, Y. Hua, P. Yang, and R. Gras, Anal. Chem. 23, 13855–13859 (2018).
(16) T. Watanabe, K. Kato, N. Matsumoto, and T. Maeda, Chromatography 27, 49–55 (2006).
(17) S. Maduskar, A. Teixeira, A. Paulsen, C. Krumm, T. Mountziaris, W. Fan, and P. Dauenhauer, Lab Chip 15, 440–447 (2015).
(18) C. Beach, C. Krumm, C. Spanjers, S. Maduskar, A. Jones, and P. Dauenhauer, Analyst 141, 1627–1632 (2016).
(19) J. Luong, Y. Hua, R. Gras, X. Yang, and P. Yang, J. Chromatogr. A 1609, 460460 (2020).
(20) J. Luong, Y. Hua, R. Gras, and R.A. Shellie, Anal. Chem. 91, 11223–11230 (2019).
Jim Luong, Yujuan Hua, Ronda Gras, and Guangyu Liu are with Dow Chemical Canada ULC in Alberta, Canada. Xiuhan Yang is with Dow Chemical China Investment Co., Ltd. in Shanghai, China. Peilin Yang is with Dow Chemical USA, Analytical Science in Lake Jackson, Texas. Direct correspondence to: luong@dow.com.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










