The (Mis)education of an Analyst
Douglas E. Raynie explores formal and informal training opportunities that may be available to educate all chemists in the fundamentals of necessary laboratory sample preparation skills.
Although sample preparation is considered an enabling technology, it is among the most vital components of an analytical scheme. Given this apparent dichotomy, where does the analyst working at the laboratory bench learn about sample preparation approaches, especially chemi-cal extractions? How is this situation exacerbated when these analysts have an undergraduate Arts or Science degree, or no degree at all; or perhaps their training is in biology, physics, or any other laboratory science? This month, we will take a look at formal and informal training opportunities that may be available to educate all chemists in the fundamentals of these nec-essary laboratory skills.
I once heard a joke along the line of “How many biologists [or insert other type of nonchemical scientist] does it take to prepare a sample?” Answer: “None, that’s what chemists are for.” While the attempted humour stems from the realization that extraction and other sample preparation techniques are chemical processes, it also points to how little attention many scientists pay to these essential steps. Further, the ubiquitous nature of sample extractions also leads them to be less than fully appreciated. After all, we find extractions in brewing coffee or tea, laundering clothing, and elsewhere in everyday living. But the problem may not just be a lack of concern about sample preparation. The issue may be a lack of sufficient training.
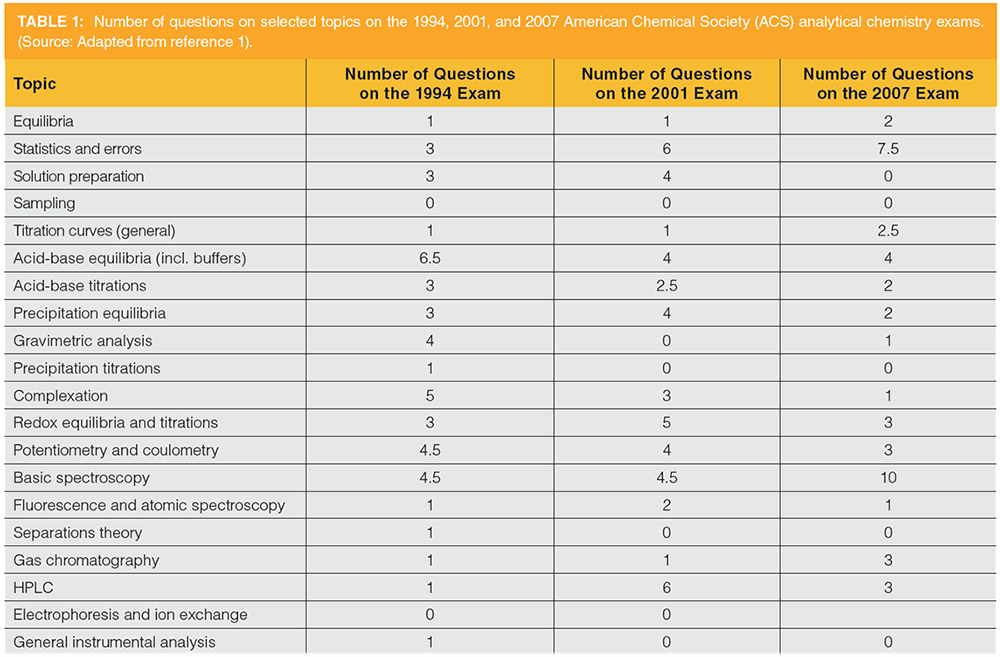
The question becomes “How do we train bench chemists, laboratory managers, regulators, and others in the performance of chemical extractions?” Analysts at all levels must have knowledge of chemical extractions as they develop new methods, consider new technologies for the laboratory, read and write standard operating procedures (SOPs) or draft regulatory methods, and so forth. There are few sources that combine a working knowledge of the performance of common extraction procedures, with an overview of new and emerging technologies, provide a basic understanding of common analytical extractions, or articulate a thorough theoretical basis for this mode of chemical separations. This situation is compounded by what is taught regarding chemical separations, including extractions, in the common chemistry curriculum. Griffiths (1) reports that, in a survey of analytical professors in the western United States, in the quantitative analysis course that typically represents the first, and often only, analytical chemistry course taken by undergraduate chemistry majors, the topic of “separation theory” is covered in an average of 1.7 lecture periods, and this mostly includes chromatography theory and the concept of distribution coefficients (discussed below), and 13% of courses do not address the topic. Furthermore, the nationally normed American Chemical Society analytical chemistry exams, summarized in Table 1, barely touch the subject. Perusal of the more recent exams does not reveal anything significantly different.
Only an occasional, somewhat specialized tome (for example, reference [2]), provides a combination of the practice, applications, basic understanding, and detailed theory of analytical extractions. Another good source of such information is the short course programme at any number of professional meetings. (For disclosure, I regularly teach sample preparation courses at Pittcon and the Eastern Analytical Symposium.) One advantage of professional short courses is the opportunity to directly interact with experts in the analytical extraction field.
Practice of Extraction
Analytical chemistry textbooks provide an overview, at best, of the practice of extraction. Additionally, laboratory technicians often have two-year degrees or bachelor’s degrees in laboratory sciences other than chemistry. Although the practice of chemical analysis is an emphasis of the curriculum of chemical technology degrees, we often must look beyond analytical education materials to get an understanding of practice. Keeping in mind that chemical extractions are not an exclusive domain of the analytical discipline, the practice of extraction is often covered in organic chemistry laboratory manuals, and most chemists, regardless of educational level, are required to complete an organic chemistry sequence.
Liquid–liquid extraction (LLE), including proper use of separatory funnels, is found in organic chemistry laboratory manuals. Solvents commonly used for extraction are presented, and although safety is mentioned, health and environmental concerns in the selection of solvents are typically not addressed, though the subject of green chemistry is becoming increasingly commonplace.
The most basic understanding of chemical extractions, in the form of distribution, or partition, coefficients is presented:
K = Corg/Caq = analyte concentration in organic phase/analyte concentration in aqueous phase. [1]
This leads to the ability to predict the ratio of solute (analyte) in the (aqueous) sample phase to that originally present in the sample phase after n number of batch extractions:
(Final solute amount)aq/(Initial solute amount)aq = (Vaq/(Vaq + VorgK)n [2]
where V is the aqueous (aq) or organic (org) solvent volume. This use of distribution coefficients makes some assumptions related to chemical potential that is beyond the scope of these laboratory manuals.
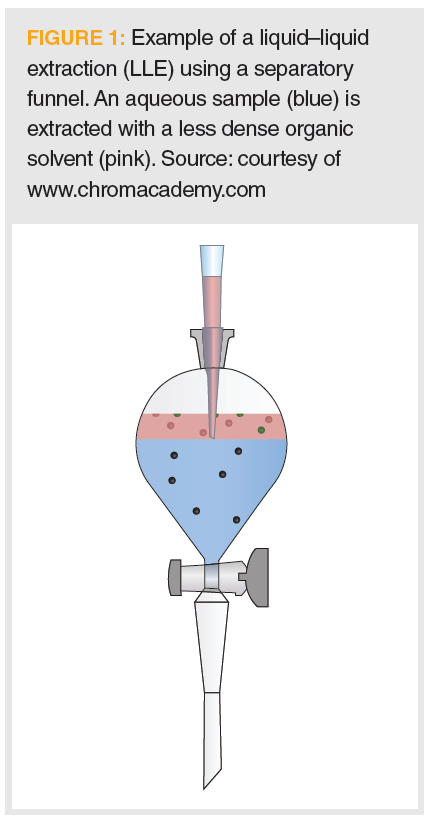
An example of a typical LLE, as presented in organic chemistry laboratory manuals, is shown in Figure 1. In this example, the (blue) aqueous sample contains three solutes (red and green spheres and squares). Based on solubility differences, reflected by the distribution ratio, the spherical molecules preferentially partition into the (pink) lighter-than-water organic extracting phase, which is then removed for subsequent work up and analysis.
The balance of the discussions is more practically focused. An overview of manipulation of pH relative to analyte pKa to protonate or deprotonate acid, amine, and related functional groups is often presented. The practice of extraction may include topics like glassware for cases of solvents more or less dense than water, washing the organic phase, salting out, and preventing or dealing with emulsion formation. Techniques other than liquid–liquid extraction are rarely presented, because most organic systems encountered in undergraduate organic experiments are solutions. Occasionally solid-phase extraction (SPE) is presented, but a comprehensive treatment of the intermolecular attractions that provide the basis of solute sorption is lacking.
In addition to the practical nature of organic chemistry laboratory manuals, the International Union of Pure and Applied Chemistry (IUPAC) (3), as well as Majors and Hinshaw (4), present guides to the terminology used in extraction and chromatographic separations. Such terms as batch vs. continuous extraction, static vs. dynamic conditions, solvent to feed ratio, raffinate, residue, extraction stage, diffusion, surface tension, adsorption, solubility, polarity scales, or isotherms are presented, and become the basis for the understanding and proper description of extractions.
Finally, although often not explicitly presented, knowledge of physicochemical parameters like polarity, volatility, molecular weight, and pKa of both the analyte and potentially interfering compounds is necessary in designing an extraction.
Basic Understanding of Extraction
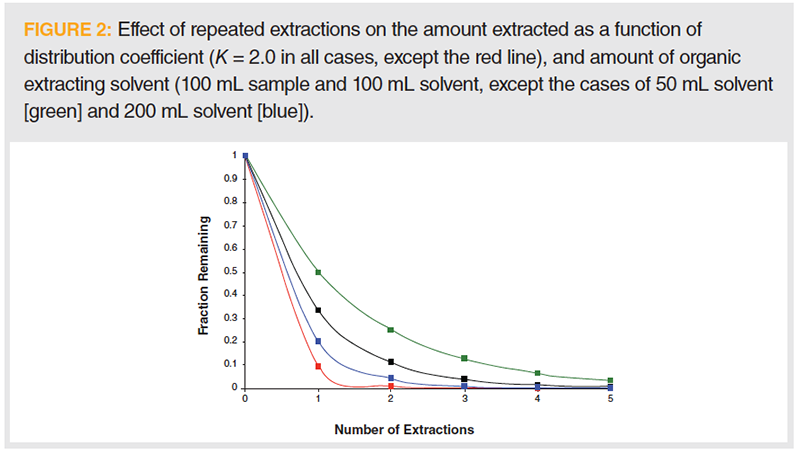
It is reasonable to assume that the next level of understanding of analytical extractions can be found in undergraduate quantitative analysis textbooks. But, as previously discussed, this assumption may be suspect. Perusal of three of the most widely used analytical texts showed that treatment of separations theory and extractions varied. Strong emphasis is placed on equilibria. This is important, because concepts like partitioning are presented in terms of equilibria. However, it should be noted that most extractions are operated away from equilibrium conditions (that is, kinetics must also be considered), and, although thermodynamic understanding is needed at the most advanced theoretical level, extractions occur through the application of heat and work in opposition to the second law of thermodynamics. Although distribution coefficients, as defined above, are presented, this information may be used to more closely determine the role of solute solubility, as measured by the partition coefficient, solvent‑to‑sample volume ratios, and number of extractions needed to isolate a given amount. For example, Figure 2 compares the quantitative nature of extractions where the distribution coefficient is 2.0 (meaning the solute is twice as soluble in the organic phase as in the aqueous sample phase) and 50 mL (green line), 100 mL (black line), and 200 mL (blue line) of organic extracting solvent is used to isolate the analyte from 100 mL of aqueous sample. This is in comparison to the red line, which demonstrates an increase in the distribution coefficient to 10 and 100 mL of organic solvent is used.
In cases where the analyte may dissociate or decompose, the distribution coefficient may be replaced by the distribution ratio, which accounts for all forms of the analyte species in each phase. For example, when carbon dioxide is dissolved in water, the distribution ratio, D, must account for CO2, H2CO3, HCO3−, and CO32− levels in each phase. With this understanding, the percent extracted, %E, is expressed as:
%E = 100D/(D + (Vaq/Vorg)[3]
Beyond this, analytical textbooks may begin to delve into SPE and related techniques with a more thorough treatment of the relationship between intermolecular attractions, solute adsorption, and sorptive phase selection. Extraction of solids, such as Soxhlet extraction, is begun to be discussed and, in some occasions, a cursory definition of advanced methods, such as supercritical fluid extraction (SFE), microwave-assisted extraction (MAE), or ultrasound-assisted extractions (UAE), may be presented. Some textbooks discuss topics like complexation and post-extraction sample clean-up.
Separations Theory Applied to Analytical Extractions
A more extensive treatment of theory in general found in the engineering literature, in graduate courses, and in specialized texts. In such training aids, treatment of the extraction mechanism may be broken down into the two major components, phase contact and phase separation. A discussion of molecular transport, typically via diffusion explained using Fick’s Laws, can be presented. Distribution ratios, rather than distribution coefficients, are preferred so that mass balance can be discussed alongside energy flow. In discussing mass balance, the potential for intentionally or unintentionally hydrolyzing or otherwise reacting the analyte is considered, as is thermal degradation. For extracting solid samples, topics like solvent wetting of the solid surface, solvent penetration into the solid particle, the impacts of a static solvent layer around the sample particle, particle-size and porosity, and extraction temperature should be a focus. Such presentations can demonstrate how particle size reduction can increase both internal transport by reducing the length of the diffusion pathway and external transport by enlarging the surface area of particle–solvent contact.
Treatment of extraction distribution coefficients or ratios can lead to Gibb’s phase rule:
F = C – P + 2 [4]
where F is the number of degrees of freedom describing the system, C, is the number of system components, and P is the number of phases. If temperature and pressure are held constant during extraction (and they generally are), the factor of two is valid, and the equation can be used to explain the function of other extraction parameters. For example, in a headspace extraction, the volume of the headspace, as well as the sample phase, is shown to be vital. In another instance, if an analyte may be protonated or deprotonated, the number of system components increases, and it can be shown that the concentration of analyte in the extracting phase depends not only on its concentration in the sample phase, but also on the pH of the sample phase.
Other advanced treatments may include interfacial tension or solubility parameters. Low interfacial tension is needed to drive mass transfer across the phase boundary and predicts initial solvent disruption and distribution in solvent-entrained solutions. When solute solubility increases, interfacial tension is generally lower, or more favourable. Other solubility treatments like the Hildebrand solubility parameter is another worthwhile discussion. The Hildebrand solubility parameter is a measure of the cohesive interaction energy of solute-solvent mixtures related to the heat of vaporization on the system. It is described by the hydrogen‑bonding ability, dispersion coefficient, and polarity and if the Hildebrand solubility parameter of a solvent is close to that of the analyte (solute) of interest, extractions become more favourable. Other, similar solvent descriptors are available. The Hildebrand solubility parameter and other treatments are the basis for various modelling simulations of chemical extractions; they are widely used in the engineering literature.
Some of the theoretical treatments (with or without a presentation of Gibb’s phase rule presented earlier), for isolating chemical compounds from the analytical sample, provide the basis for specific, often more advanced extraction techniques like SPE, solid‑phase microextraction (SPME), related sorption‑based technologies, SFE, MAE, UAE, or pressurized‑fluid extraction. Practice and detailed descriptions of the basis for these techniques are usually found in more specialized literature.
Conclusion
While knowledge is power, unfortunately the formal education of most analysts centres on education in advanced analytical techniques, good laboratory practice, SOPs, regulatory compliance, laboratory safety, and other items. Common laboratory procedures, like extractions, are often excluded from the educational process. Awareness and knowledge of advanced extraction and sample preparation procedures is even more scarce. In this column, we presented an overview of the type of information that should be included in training protocols, and discussed where this information can be found.
References
- P.R. Griffiths, Anal. Bioanal. Chem. 391, 875–880 (2008).
- R.E. Majors, Sample Preparation Fundamentals for Chromatography (Agilent Technologies, 2013).
- L.S. Ettre, Pure and Appl. Chem. 65, 819–872 (1993).
- R.E. Majors and J. Hinshaw, LCGC North Am. 34(S2) (2016).
Douglas E. Raynie is “Sample Prep Perspectives” editor. He is a Department Head and Associate Professor at South Dakota State University, USA. His research interests include green chemistry, alternative solvents, sample preparation, high‑resolution chromatography, and bioprocessing in supercritical fluids. Direct correspondence to: amatheson@mjhlifesciences.com

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)



