Technology and Application Highlights of HPLC 2007
LCGC North America
HPLC 2007 was held in Ghent, Belgium in June. Last month, columnist Ron Majors summarized some the important column developments as well as other Symposium highlights. This month, he winds up coverage with additional highlights in the areas of technology and applications. Among the topics covered are stationary phase preparation and characterization, multi-dimensional and comprehensive LC, temperature studies, detectors and an application overview.
The 31st International Symposium on High Performance Liquid Phase Separations and Related Techniques, which alternates between Europe and North America, was held for the first time in Belgium at the International Convention Center, Ghent, Belgium June 17–21, 2007. More affectionately known as HPLC 2007, the symposium is the premier scientific event for bringing together the myriad techniques related to separations in liquid and supercritical fluid media. Cochaired by Professors Patrick Sandra of Ghent University (Ghent, Belgium) and Jacques Crommen, University of Liege (Liege, Belgium), the symposium assembled just under 1200 scientists from a total of 45 countries. This number did not include vendor representatives from over 70 exhibitors for the three-day instrument, software, and consumables exhibition.

The five-day-plus event had a total of 122 oral presentations, many presented during simultaneous sessions, 800 posters (the largest for this meeting to date) in sessions with 28 themes. Last month (1), I reported on the honorary sessions, the best poster and other award winners as well as the highlights of column technologies of HPLC 2007. This month, I will continue the report of the meeting with a summary of the plenary lectures, technology and applications topics.

Ronald E. Majors
Plenary Lectures
Opening Ceremony plenary lectures, presented on the first evening of the Symposium, are supposed to be leading-edge, thought-provoking presentations that inspire attendees to think beyond the box. This year, two notables gave plenary lectures that were oriented towards proteomics and systems biology. Barry Karger of Northeastern University, Boston, Massachusetts, was the first plenary lecturer and gave an interesting presentation on ultratrace liquid chromatography–mass spectrometry (LC–MS) proteomics analysis, an area in which he has been active for some time. As life science researchers delve deeper and deeper into the proteome, looking for better biomarkers of disease, techniques such as LC–MS may replace (or at least supplement) traditional measurement techniques such as ELISA and CA125 (cancer antigen-125) and PSA (prostate-specific antigen) and other cell-based markers. Karger's laboratory has been investigating new methods to uncover biomarkers for such common tests as Pap Smears using a small number of cells. Thus, high sensitivity methods are required. For this reason, his latest work involves the use of 10-μm i.d. porous layer open tubular (PLOT) columns because as the column internal diameter goes down, the low flow rates required provide fewer solvent clusters and higher ionization efficiency in MS. In his experimental setup, he initially uses on-line, in-series nanobore columns, first with a C18, followed by a cation exchanger, and finally the polystyrene–divinylbenzene (PS-DVB) PLOT column. Starting with 12,000–15,000 cells containing about 1–4 μg of protein and performing a laser-capture microdissection, cell lysis, SDS-PAGE, and in-gel digestion in that order, he is able to identify 1867 unique proteins and can narrow down the total numbers of proteins of interest to four specific proteins that may be disease markers for cervical cancer. These are currently under study. These proteins have been detected at the femtomole and attomole levels.
The second plenary lecture was presented by Jan van der Greef, TNO Systems Biology, The Netherlands. Unlike Karger's more practical presentation, van der Greef discussed systems biology from a high level. Although there are many definitions of systems biology, some sources consider it a field of study, particularly, the study of the interactions between the components of biological systems, and how these interactions give rise to the function and behavior of that system (for example, the enzymes and metabolites in a metabolic pathway). It ties together many disciplines ranging from measurement and analysis to infomatics. For systems biology, there are many cross-compartment relationships (for example, plasma versus tissue) and correlation networks that have to be dealt with. Van der Greef discussed the concept of empirical medicine versus biomarker-guided medicine and traditional medicines (for example, Chinese traditional medicine) versus Western medicines and how these can interact with possible synergies. He even talked about mind–body interactions and personalized medicine as areas to be explored.
On Monday morning, Jeremy Nicholson of the Imperial College, London, UK, continued the systems biology theme but looked at it from the postgenomic viewpoint to help understand adverse drug reactions and the molecular basis of human disease. He approached it from a measurement–statistical viewpoint using tools such as nuclear magnetic resonance (NMR) spectroscopy (proton and fluorine), LC–NMR, LC–MS, and statistical heterospectroscopy in which hybrid NMR–MS spectra can be reconstructed and combined with chemometrics to interrogate all this information to give direct diagnostic information and aid in the detection of biomarkers and in the integration of metabolic data with other "omics" sets. He also talked about personalized healthcare in which the development of expert systems for individual prediction of drug toxicity and metabolism could be possible. Using human gut microbial metabolic profiles, he was able to see distinct differences in various races and even in geographical distributions of the same race.
The second plenary lecture for Monday morning was delivered by Robert Kennedy (University of Michigan, Ann Arbor, Michigan), after the original lecturer had flight problems getting to the Brussels airport. Kennedy talked about high-throughput separations mainly around the use of the microfluidic format and electrophoretic separations, parallel separations and the incorporation of sampling and sample preparation into the overall process. His group has been developing on-line analyzers for multiple cell cultures where samples are taken continuously and injected into a multichannel capillary electrophoresis (CE)-based system for subminute separations. Their most recent system was a 48-channel circular design using multilayer soft lithography. Using a peristaltic poly(dimethyl siloxane) (PDMS) pump, they are able to manipulate samples before the CE separation. Their test system was an assay to detect G protein-coupled receptor (GPCR)-stimulated G protein GTPase activity in cell membranes. They were able to separate BGDP and BGTP in all 48-channels.
Stationary Phase Preparation and Characterization
Researchers are always finding new and better ways to prepare stationary phases in HPLC. In Table I, new phase design was among the top of new technologies.

Table I: HPLC 2007 papers presented by technology or technique
An interesting approach to stationary phase synthesis was presented by Norma Scully, winner of the 2006 Horvath Prize. In her award address, Scully and her colleagues used supercritical carbon dioxide as a reaction media in the preparation of silica hydride intermediates. In recent years, there has been considerable interest in silica hydrides as intermediates in the preparation of chromatographic stationary phases via a silanization–hydrosilylation approach. She exploited the properties of supercritical carbon dioxide such as solvating power, lower toxicity, and enhanced diffusivity to react organosilanes. In her comparisons to regular organosilane synthesis using organic solvents, this supercritical carbon dioxide reaction medium provided faster reaction with the same degree of completeness or better. For example, in the preparation of a silica hydride intermediate using a 3-μm silica gel with surface area of 194 m2 /g, she was able to obtain a surface coverage of 75% of the silanols (6.0-μmol/m2 ) in just an hour versus 100 h required for a toluene solvent system. Similar results were obtained for a C18 phase and a pentafluorophenyl propyl (PFPP) phase. She used solid-state NMR and thermal analysis to characterize her bonded phases. She also employed the NIST SRM 869 test (see the following) to characterize the shape selectivity of her bonded phases.
A couple of papers stood out as new approaches to study column packings. Inverse size-exclusion chromatography (SEC) is a standard technique to determine the interstitial volume or external porosity inside a packed column or a monolithic column. A poster presentation by D. Cabooter and colleagues from the Vrije Universiteit Brussel and Universiteit Gent developed a new method that is intrinsically simple and useful for investigating the porous structure of a reversed-phase packing. The method is based upon the total blocking of the micro- and meso-pores by filling the porous support with a hydrophobic solvent. The strong interaction of the latter with the hydrophobic coating inside the pores keeps the solvent in position during the subsequent measurements. With the pores of the stationary phase material completely inaccessible for any type of polar molecule, the method allows interstitial void measurements using small molecular weight molecules rather than the large molecular weight molecules needed in inverse SEC. Small molecules are much better probes because they can penetrate every part of the interstitial volume and provide very accurate determination of the external porosity. The method was used to compare the porous structures of several sub-2-μm porous packing materials.
The United States National Institute for Standards and Technology (NIST) has been using various tests to study the surface characteristics of chemically bonded phases, especially with regard to shape selectivity. This year, C. Rimmer and coworkers (NIST, Gaithersburg, Maryland) presented examples of the NIST SRM 869 SP structure test to compare monomeric, polymeric, and self-assembled monolayer phase prepared on silica plates (for spectroscopic investigations) and columns (for chromatographic investigations) that were subjected to several diagnosis. Using their test conditions, under normal conditions if αTBN/BaP is greater than 1.7 (where TBN is tetrabenzonaphthalene and BaP is benzo[a]pyrene), this indicates a monomeric phase and if the value less than 1, this indicates a polymeric phase. There appears to be a temperature effect on shape selectivity. For example, at 5 °C, a monomeric phase looks like a polymeric phase while at 60 °C, compounds are eluted from the polymeric phase in the "monomeric" order. The workers used various spectroscopic tests to study these phases directly. Atomic force microscopy (AFM) did not show any differences between a C18 and a similarly prepared C30 but this was due to lack of resolution. Dichroic infrared and X-ray photoelectron spectroscopy (XPS) show both chains sticking out at a 45° angle. Perfluoro C18 phases show distinct behaviors in methanol versus the hydrocarbon C18 phases as the "negative" polarity of fluorine attract the OH (or water and methanol) presenting a methyl surface to test compounds instead of the polar grouping in usual reversed-phase separations. With the perfluroro stationary phase, the behavior of solutes is considerably different in methanol and acetonitrile.
Multidimensional and Comprehensive Liquid Phase Separations
As samples become more complex, even with the best and most efficient columns available, the peak capacity of a single column is no longer sufficient to resolve all the peaks to baseline. Peak capacities of 1000 can be achieved with a single column but to handle samples with thousands and tens of thousands of components, multiple columns are needed. The total peak capacity of a multidimensional system is equivalent to the peak capacity of the initial column multiplied times the peak capacity of the second column. This statement is true only if the two chromatographic modes are orthogonal — totally different mechanisms of separation.
Multidimensional chromatography has been around for many years but has only achieved minimal success. With high-speed switching valves controlled by computerized systems, techniques such as heart cutting and flow diversion to eliminate uninteresting peaks are becoming more commonplace. Comprehensive LC, termed LC×LC, is a technique that also is attracting interest. In this approach, every single fraction from the first dimension is switched onto a second dimension to be further separated and identified. This switching is accomplished without shutting down the flow of the first dimension column by somehow storing the effluent from column one in a loop or packed bed and switching that storage device onto the second column once it has finished its analysis. The implication here is that the second dimension must be extremely fast so monoliths or short-packed columns capable of subminute separations are used. Readers who are interested in more details can consult reference 14.
Table I showed that multidimensional, comprehensive, and column switching techniques are gaining recognition. In fact, an entire oral session was devoted to comprehensive and multidimensional techniques. This session on the final day was led off by Prof. P. Schoenmakers of the University of Amsterdam, The Netherlands. Schoenmakers' presentation discussed LC×LC. He was quick to point out that this technique is not a stop-flow or off-line (fraction collection) technique but is performed continuously. He discussed the relative advantages and disadvantages of using different column lengths and diameters in the first and second dimension. His LC×LC system uses two interface loops (and sometimes another second dimension column) to permit a longer second dimension timescale to give a better separation and require fewer cuts from the first dimension. The optimum peak capacity can be achieved if one can make a minimum of four cuts from the first dimension peak. The less frequently one samples the first dimension, the greater the loss of peak capacity.
Some applications of comprehensive liquid phase chromatography at HPLC 2007 were the analysis of phenolic acids in herbs and wines, pHEMA-co-BA (LC × SEC), lignosulfonates in plants (SEC × ion-pair reversed-phase LC), polymer consisting of polycondensate of butanedioic acid and diisopropylamine (reversed-phase LC × SEC, normal-phase LC × SEC, normal-phase LC × reversed-phase LC), chiral and achiral pharmaceuticals, lemon oil extract (normal-phase LC × reversed-phase LC), fish oil extract (reversed-phase LC × reversed-phase LC), sulfonamides and impurities (reversed-phase LC × reversed-phase LC), psoralens and coumarines (supercritical fluid chromatography [SFC] × reversed-phase LC), vegetable oils (NARP × HPLC), and biomarkers (LC × LC).
To achieve even more selectivity when attempting to analyze small amounts of derivatized branched aliphatic amino acid enantiomers in body fluids, one paper used comprehensive micro two-dimensional chromatography with an achiral microbore-C18 column in the first dimension and an ion-exchange chiral stationary phase in the second dimension. The fully automated systems had a lower limit of detection of 500 amol when using a fluorescence detector.
Temperature Studies in HPLC
As noted in Table I, one of the "hot" areas at this year's meeting was the role played by temperature in HPLC. Two complete oral session and many posters were devoted to temperature studies, mainly with high temperature being the focus. In a kickoff lecture in the second session, G. Rozing of Agilent Technologies, Waldbronn, Germany gave an overview of the requirements and outcomes for high-pressure and high-temperature operation. Today's HPLC equipment operates up to 120 °C and 1000 bar to force mobile phase around sub-2-μm particles. Perhaps next will be sub-1-μm particles and even long monoliths. Thus, even higher pressures may be required, 1500 or even 2000 bar. It is already known that high pressures cause deformation of the stationary phase and even the column tubing. One must also consider the effect pressure has on solvents and solutes. It is known that pressure increases melting points, viscosities, and diamagnetism. The velocity profile changes — leading to band broadening. Due to frictional heating, the temperature increases with the square of the pressure change. Somehow, we must get rid of this heat. The mobile phase and the adjacent column wall will have a temperature gradient, resulting in changes in plate height. If separations are run isothermally, at least 10 min will be needed to reequilibrate the column after the separation, more if temperature programming is used. With a high-pressure gradient, the column will never be in thermal equilibrium. Rozing referred to the Guiochon and Martin paper (12) for those who were interested in more detail on the effects of pressure on physiochemical measurements.
M. Kele of Waters, Milford, Massachusetts gave a lecture on the practical and theoretical performance of sub-2-μm columns with temperature. Higher temperatures yield faster separation due to lower viscosities (and, thus, lower pressure), reduced retention, higher optimal linear velocities, and improved diffusivities. Less mobile phase is required at high temperature because retention is reduced. Selectivity is affected by temperature. For high-temperature operation, mobile phase preheating is required. Thermal stability of analytes is required. Increased temperature changes the ionization constant. At 90 °C, 1.7-μm particles do not show a van Deemter curve much different from 30° while at 90 °C particles have a similar van Deemter curve to 5-μm particles at 30 °C. These effects relate to increased mobile phase diffusivity and result in reduced retention factors although the optimum velocity also increases. Earlier in the same session, U. Neue, also from Waters, gave a talk on ultrahigh-pressure LC, in which the advantages of high pressure over high temperature were given. He showed a number of new columns, one based upon a high-strength silica base material that shows enhanced retention of polar compounds. Another column was suitable for HILIC, while a third was a wide-pore bridged ethyl hybrid particle recommended for peptides and proteins.
In separate lectures, T. Teutenberg and coworkers from the Institute of Energy and Environmental Technology, Duisberg, Germany and from the Carl von Ossietzky University in Oldenburg, Germany, and R. Smith and coworkers from Loughborough University, Loughborough, UK talked about available stationary phases for high-temperature operation. Columns that have been advocated for high temperature use are metal oxides such as zirconia (ZrO2), titania (TiO2) and coated metal oxides (for example, polybutadiene, PBD), PS-DVB polymers (160 °C), and graphitzed carbon (180 °C). Columns based upon silica with polymer-encapsulated phases or heavily endcapped can be used above 100 °C, but most bonded silica columns are not suited for temperatures in excess of 100°. For example, Smith's group found that XTerra (Waters) was limited to 90 °C but they tested X-Bridge Phenyl to 200 °C. The German group did some stress testing and found that the ZrO2-PBD (Zirchrom, Anoka, Minnesota) could be used from room temperature to 200 °C and gave little bleed after the first run, as measured by evaporative light scattering detection (ELSD) and UV detection. The PDRP-S (Hamilton, Reno, Nevada), a PS-DVB polymer, gave some bleeding initially but was able to work up to 200 °C without further bleed.
They found that most mobile phases can be used throughout the temperature range (room temperature to 200 °C) but with tetrahydrofuran–water, there are some temperature areas of immiscibility called the miscibility gap. To keep solvent from degassing or boiling, a backpressure of at least 22 bar should be applied to a UV detector. For the instrumentation, they recommend solvent preheating and a postcolumn cool down option because the temperature can affect the detector noise. In their work, they found no evidence of analyte instability problems as a function of increased temperature. The authors stated that the residence time is quite curtailed in many of the common columns — especially very short ones. In the future, the workers see more temperature gradients being used to speed analyses, and they could be combined with solvent gradients. They used of pure water as an eluent that allows the use of isotope ratioing mass spectrometry. Smith's group was able to use the flame ionization detector when pure water was used as a mobile phase at elevated temperature. If D2O was used as a mobile phase, then NMR could be used for detection.
Applications Highlights
As can be seen in Table II, protein and peptide separations and proteomics again dominated liquid-phase applications. This year, the discovery of biomarkers, both large and small molecules, for disease diagnosis was a popular topic. Pharmaceutical companies are looking for tools that will be able to measure and predict the efficacy of candidate drugs in shorter times and with less expensive clinical trials. Disease-specific biomakers can speed up the process of drug discovery. For protein-based biomarkers, the use of multidimensional LC coupled with MS and MS-MS is becoming a standard technology. With even more techniques now available for the removal of high-abundance proteins from human plasma, trace levels of proteins are more readily detectable, although some presenters noted that sometimes small concentrations of desired proteins can tag along with the high-abundant proteins that are removed.
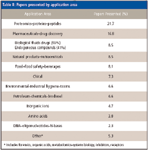
Table II: Papers presented by application area
As usual, separations of pharmaceuticals (bulk drugs, tablets, and other formulations), including impurity profiling, had a strong showing with presentations from most of the major pharmaceutical companies. Furthermore, the analysis of drugs, drug metabolites, and endogenous compounds in biological samples and clinical chemistry continued to have a large following. Many poster presenters were involved in discussions with conferees about their experimental approaches to solve these problems.
The number of applications papers using CE and related techniques was quite strong again this year (Table I). This technique, which has slowed commercially, has been used in solving many problems in the life sciences, environmental science, metal analysis, and many other complex problems. The use of nonaqueous CE for chiral compounds and other pharmaceuticals continues to grow. With two-dimensional separations now gaining favor, some workers reported the successful combination of CE or micellar electrokinetic chromatography (MEKC) with ion chromatography or reversed-phase chromatography. These two approaches were truly orthogonal in the mechanisms. MS is a favored detection technique with electrophoretic separation techniques also (Table III).
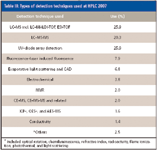
Table III: Types of detection techniques used at HPLC 2007
I was surprised at the lack of emphasis on capillary electrochromatography (CEC) again this year (Table I). At one time, CEC sessions filled the halls, and at HPLC 2007, there were only a couple of oral presentations and a handful of poster papers that stressed this technique. Perhaps CEC has not lived up to its promise; for the most part, there have not been too many separations problems solved by CEC that could not be accomplished by HPLC or CE alone. It will be of interest to see if that "killer application" can be found in the future.
Detection Techniques
As can be seen in Table III, LC–MS and LC–MS-MS were again the dominant detection methods used in the oral and poster presentations at HPLC 2007. When combined with the various electrophoretic techniques that were combined with MS detection, almost 50% of all papers that provided information on detection techniques used some form of MS. After MS, as might be expected, UV detection, especially diode array detection was the second favored detection technique, mostly in application examples.
ELSD has been gaining in popularity. This detection method is universal, can be used with gradients, and might be more sensitive than refractive index for a variety of applications. But charged serosol detection (CAD) based upon corona discharge might replace ELSD, as it appears to be an order of magnitude more sensitive than ELSD. CAD is also an evaporative technique and is based upon the charging of aerosol-borne analytes by nitrogen gas and corona discharge with subsequent measurement of the charged nonvolatile analyte particles by a high-sensitivity electrometer. The increasing number of applications at HPLC 2007 (and not just by the manufacturer) are indicative of the acceptance of the CAD. Applications of CAD in pharmaceutical analysis were the most prevalent where it was found to have a more consistent response for different drug substances.
HPLC 2008 is Next
The next major symposium in this series, the 32nd International Symposium on High Performance Liquid Phase Separations and Related Techniques (HPLC 2008), moves back to the United States and will be held in Baltimore, Maryland May 10–16, 2008. This will be the second time this series has been held in Baltimore, and co-chairmen of this upcoming event will be Prof. Georges Guiochon of the University of Tennessee and Oak Ridge National Lab and Prof. Steve Jacobson (Figure 1), Indiana University. For more information consult the official website http://www.hplc2008.org/.

Figure 1: Prof. Steve Jacobsen making his pitch for HPLC 2008 at the HPLC 2007 meeting in Belgium.
Acknowledgments
I would like to acknowledge the contributions of my Agilent colleagues: summaries from Thomas Doerr, Bernd Hoffman, and Ulrik Wittek of Waldbronn, Germany and detailed notes from Maureen Joseph, and Bill Barber of Wilmington, Delaware. I also would like to give a special thanks to Professor Carol Collins of State University of Campinas, Sao Paulo, Brazil for her excellent summary of many of the columns talks that I was unable to attend. Finally, last but not least, I want to thank the Poster Committee members who worked very hard reviewing (and sometimes re-reviewing) the posters at HPLC 2007.
Ronald E. Majors
"Column Watch" Editor Ronald E. Majors is business development manager, Consumables and Accessories Business Unit, Agilent Technologies, Wilmington, Delaware, and is a member of LCGC's editorial advisory board. Direct correspondence about this column to "Sample Prep Perspectives,"LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgc-mag.com
References
(1) R.E. Majors LCGC 25(9), 920–942 (2007).

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.












