A Systematic Study of Achiral Stationary Phases Using Analytes with a Molecular Diversity Model
LCGC Asia Pacific
The authors characterized 12 SFC achiral stationary phases with 60 compounds from four chemical classes to establish guidelines for th rational selection of the optimum stationary phase for separations.
Using a molecular diversity model to choose test probes from a chemical library, guest authors Ray McClain and Matt Przybyciel characterized 12 supercritical fluid chromatography (SFC) achiral stationary phases with 60 compounds from four chemical classes — amines, amides, alcohols and carboxylic acids — to establish guidelines for the rational selection of the optimum stationary phase for separations. Efficiency, selectivity and peak shape were used as the evaluation criteria.
The use of supercritical fluid chromatography (SFC) as a separation technique continues to grow (1–6). SFC offers a number of advantages to chromatographers for both analytical and preparative separations. These advantages include the "green" aspect of using carbon dioxide as a mobile phase, the ease of isolation of purified components from preparative separations, and decreased analysis times because of the high diffusivity and low resistance to mass transfer encountered in SFC. For many years, SFC has proven extremely useful for chiral preparative separations (7–12). Achiral SFC separations utilize stationary phases from "normal-phase" high performance liquid chromatography (HPLC), such as unmodified silica, diol, amino and cyano (13). During the past 10 years, a number of commercially available stationary phases have been developed specifically for SFC applications (14). However, not all of these columns are well-suited for all structural classes of compounds, presenting a bottleneck when arrays of chromatographic data must be acquired and interpreted to find a suitable match. Recently, chemometric approaches have been developed to predict retention and selectivity for a variety of stationary phases used for SFC (1,15–19). These approaches can be a useful tool for column selection with a particular set of analytes. Despite this, many chemometric approaches don't provide any guidance regarding peak shapes of the separated components. In addition, the chemometric models generally require physical or chemical information for the compounds of interest, such as excess molar refraction, dipolarity and polarizability, hydrogen bond donor (acidity), hydrogen bond acceptor ability (basicity) and McGowan's volume (19); for many analytes of interest, information such as this is not readily available.
Separations of Chemical Libraries
Many pharmaceutical laboratories are involved in the characterization and purification of large chemical libraries that are employed in high-throughput screening for new drug development. These libraries are particularly challenging in the chemical diversity that they represent (20,21). Library molecules may contain a variety of different functional groups and may differ substantially in properties such as pKa, hydrophobicity, molecular weights and molecular surface area, to name just a few. SFC–mass spectrometry (MS) has been shown to be a useful tool for the purification of chemical libraries (3,20,22–25); however, the selection of the appropriate stationary phase for the separation of chemicals in the library carrying out these separations can be difficult, especially with the variety of SFC stationary phases that have recently been commercialized (14). Furthermore, most chromatographers performing purifications prefer to avoid using mobile phase additives, such as amines, because it complicates compound recovery and can lead to product degradation. This poses a unique challenge for chromatographers performing purifications on a vast array of molecules. Chromatographers separating chemical libraries would like chemical compound selectivity, as well as good peak shapes, to optimize their purifications without the use of mobile phase additives. Ideally, one would like to have a set of screening stationary phases that the chromatographer could employ to separate a wide variety of molecules containing various functional groups. To this end, we would like to identify a limited set of stationary phases that can separate a wide variety of molecules and maintain good peak shape without mobile phase additives.
Utilization of a Molecular Diversity Model
We used the large and structurally diverse chemical building block library available at Merck (Whitehouse Station, New Jersey, USA) to help identify a set of stationary phases. We queried this collection of chemicals to obtain representative compounds in four distinct functional group classes — carboxylic acids, amines, alcohols and amides. These four distinct functional group classes are viewed as important reactive groups for the synthesis of larger molecules. We identified 15 chemicals within each functional group class with maximal Tanimoto dissimilarity (26) using Merck's in-house developed software. These 15 compounds represent diverse chemical space for each chemical class based upon the combination of the large number of compounds in the building block collection and the computational calculated molecular diversity model. The structure of these proprietary compounds, serving as the test probes in this study, will not be disclosed but an example collection of commercially available amines with structures shown will be displayed later in the article. Figure 1 is an example of a screen shot from the custom written "Small Molecule Browser" software used to identify structurally diverse reagents within each functional group class. This projection is a two-dimensional nonlinear map of the ECFP4-derived Tanimoto similarity matrix (26). In rough terms, and allowing for distortions in the projection, molecularly similar compounds end up grouped together and molecularly different compounds tend to be farther apart. The red dot in the upper right quadrant was randomly selected as a starting point and the software was instructed to find the 14 most diverse carboxylic acids, relative to the starting point. The resulting selections are highlighted in blue.

Figure 1: Example of a screen shot from the custom-written "Small Molecule Browser" used to identify structurally diverse reagents and analytes within each functional group class. This projection is a two-dimensional nonlinear map of the ECFP4-derived Tanimoto similarity matrix.
Figure 2 highlights the many calculated parameters used in arranging the carboxylic acid collection into its multidimensional chemical space. We did not elect to use filters restricting the range of certain molecular parameters within a specific range to ensure the most random and diverse collection as possible. The figure displays the extensive range of each parameter used. The same molecular parameters were also used for the amine, alcohol and amide chemical classes and resulted in the selection of our test chemicals for each chemical class.

Figure 2: A typical screen shot highlighting the large number of molecular parameters, specified as row headers on the left of the image, used to arrange the functional classes into their respective chemical spaces. The vast range of each of the parameters is highlighted by red bars in each of the lines representing an individual molecular parameter in the image. The lighter red bars signify a smaller number of building blocks or analytes contained in the collection possessing a specific magnitude of the molecular parameter specified, and an intense red bar signifies a greater population of analytes possessing that magnitude of the specified parameter. Such tools were used to provide a rational selection of test probes for the SFC columns.
The Selection of the SFC Conditions and Stationary Phases Evaluated
The 15 compounds for each of the four chemical classes were analysed on seven different achiral stationary phases, many of which were in their endcapped and nonendcapped forms (listed in Table 1). A silica column was included as one of the seven, because this column has been extensively used for achiral SFC separations (13). An endcapped silica column (TMS) was also evaluated, but coverage of the polar surface of the silica virtually eliminated the retentive capabilities of the material, as expected, so it was eliminated early in the study. All of the phases evaluated were bonded to the same 5-μm, 350-m2/g, and 120-Å pore size base silica and the packed columns had dimensions of 250 mm × 4.6 mm. To maximize the interaction with the stationary phase, isocratic conditions were selected (27). As indicated by the column temperature of 25 °C, the study was performed in the subcritical state to minimize fluid compressibility and thus minimize density changes along the column that impact diffusion coefficients (19). All chromatographic data presented and discussed in this study were acquired using the following chromatographic conditions:
- Flow rate: 3 mL/min
- Mobile phase: 90% CO2, 10% methanol
- Back pressure: 150 bar
- Column temperature: 25 °C
- Detection: UV absorbance at 214 nm coupled with positive atmospheric-pressure chemical ionization (APCI) MS for compound identification
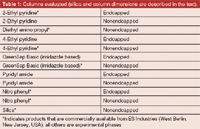
Table 1: Columns evaluated (silica and column dimensions are described in the text).
Carboxylic Acids
The 15 carboxylic acids presented by our diversity model were analysed on our column set. Of the columns tested, the 2-ethyl pyridine (EP) and GreenSep Basic columns provided the best results for the carboxylic acid test probes. Both stationary phases produced acceptable peak shape for all of the peaks on the endcapped EP, nonendcapped EP, endcapped basic and nonendcapped GreenSep Basic columns. The half-height peak efficiency values in plates per column for each peak in a specified chromatogram were averaged together to give one average efficiency per column. The values were 7770, 11998, 8489 and 8864 plates per column, respectively. A chromatogram of the carboxylic acid mixture on the endcapped and nonendcapped EP column is shown in Figure 3. The nonendcapped EP column exhibits additional selectivity as evident by the separation of critical pair peaks 10/11 and 3/6, as well as additional retention as evident by increased retention on all peaks (except peak 7). The nonendcapped version of the GreenSep Basic column did not demonstrate a change in selectivity or an increase in retention when compared to its endcapped version, as shown in Figure 4. The absence of increased retention for the nonendcapped GreenSep Basic column compared to its endcapped version suggests the free silanols are not able to compete with the imidazole for interacting and retaining the acidic test probes as experienced with the EP column. This is supported by the increase in pKa of imidazole compared to pyridine, 7.0 to 5.25, respectively. This behaviour was very interesting and requires further investigation. In addition, we discovered that diethyl amino propyl (DEAP) showed excessive retention for the acids (presumably because of the involvement of an ion-exchange retention mechanism).
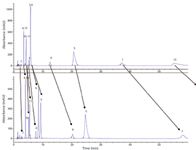
Figure 3: Carboxylic acid mix analysed under standard chromatographic conditions on the endcapped (top) and nonendcapped (bottom) 2-ethyl pyridine column.
The study found that the nonendcapped EP is the column with optimal performance for the analysis of carboxylic acids. Previous studies have reported excessive retention of acidic compounds on the EP column (3). The carboxylic acid test probes used in the present study were largely unaffected by this phenomena, but presumably the use of an endcapped version of the column could be helpful in cases where retention is unacceptably long.
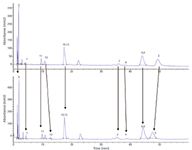
Figure 4: Carboxylic acid mix analysed under standard chromatographic conditions on the endcapped (top) and nonendcapped (bottom) Basic column.
Alcohols and Amides
The DEAP column provides excellent peak shape for the alcohols, as shown in Figure 5, even for compound 1 eluted at 25 min (see insert). The average half height peak efficiency for the alcohol test mix analysed on the DEAP column was calculated to be 9775 plates per column and the average USP tailing factor was calculated to be 1.19. This is a small but diverse data set for alcohols, and the DEAP column appears to have a vast degree of selectivity for this data set. The extremely efficient peaks may be a result of this column possessing only one retention mechanism, hydrogen bond accepting capabilities. The ideal selectivity, controlled retention and elution, and extremely efficient peaks make the DEAP column the preliminary column of choice for alcohol analysis.

Figure 5: Alcohol mix analysed under standard chromatographic conditions on the nonendcapped DEAP column.
All columns tested gave acceptable results for the chemical diverse amide compound test set. The best column for the amide compound set was found to be the nitro phenyl nonendcapped column, as shown in the chromatogram in Figure 6. This column provided excellent peak shape with an average USP tailing factor of 1.34 and good selectivity for the amides analysed. The peaks are very efficient with an average half height efficiency of 9279 plates per column with peak 9 providing good peak shape, with a half height peak efficiency of 9304 plates per column at 20 min.

Figure 6: Amide mix analysed under standard chromatographic conditions on the nonendcapped nitro column.
Amines
The SFC separation of amines is particularly challenging without the use of additives. The results for the amine test mixture varied greatly. Some of the columns tested produced very poor peak shape, had very poor selectivity and had excessive retention, and of these some exhibited both poor peak shape and poor selectivity. The GreenSep Basic nonendcapped column showed the best peak shape for most of the amines analysed, as shown in Figure 7, with the average USP tailing factor being 1.65 vs. the average tailing factor of 2.31 observed for the amines analysed on the endcapped version of the column. An additive such as diethyl amine could be added to the cosolvent in an attempt to sharpen peak shape and minimize further tailing. The use of additives is out of the scope for this article but Blackwell and Stringham (28) cover this topic more comprehensively. In our amine compound mixture, eight of the compounds are primary amines, suggesting that there would be little hydrogen bond accepting character displayed by these compounds on the GreenSep Basic column. The amine test mixture exhibited longer retention on the nonendcapped GreenSep Basic column, suggesting strong π-π interactions as all but one of the amine test mixture compounds possess aromatic systems. One of the poorest columns for amines was found to be silica with only one of the 15 amine compounds in our mixture eluted within a 60-min chromatographic analysis.

Figure 7: Amine mix analysed under standard chromatographic conditions on the endcapped (bottom) and nonendcapped (top) Basic column.
The structures of the Merck compounds used in this study could not be disclosed so a collection of commercially available amines (listed in Table 2) was selected to serve as an operational test for our approach of column assignment. Figure 8 shows a chromatogram of these commercially available amines analysed on the nonendcapped GreenSep Basic column according to the standard conditions described earlier in this article. The half height peak efficiency along with USP tailing factors for all seven of the commercial amine mixture components are shown in Table 2. Several of the components have a tailing factor greater than 2; however, all components were eluted in less than 12.5 min. Even with greater tailing factors, for some components this separation would be useful for preparative chromatography, especially considering that the conditions were isocratic and no additives were used. This mixture provides an operational test for a chemical diversity model. Based on our amine diversity mixture and our commercially available amine mixture, we believe that the nonendcapped GreenSep Basic column is the preliminary column of choice for the analysis of amines.

Table 2: Commercially available amines used as test probes.
Chromatographic Summary
One of the complexities routinely discussed about achiral SFC revolves around identification of a single stationary phase to resolve the majority of sample mixtures encountered in our laboratories. This desire could stem from the common application of C18 stationary phases in reversed-phase applications and the vast success encountered with its use. With the study presented in this column instalment, we hope to limit the experimental work necessary for column screening and data interpretation — there does not appear to be a current universal stationary phase for achiral SFC applications. Figure 9 displays the data generated for all four chemical classes on the nonendcapped nitro phenyl column in this study. The highly variable results for the four classes on this single column summarize the need for development of a universal phase for achiral SFC. The amide chromatogram displays controlled retention, controlled elution, efficient peaks and a vast degree of selectivity, making this phase the preferred suggestion for the analysis of amides. The carboxylic acid chromatogram reveals broad, inefficient peaks. This class of compounds also displays excessive retention as evident from the peaks eluted at 50–60 min and even several compounds that were not eluted from the column. The alcohol chromatogram displays the worst peak shape of all four classes of compounds on this column. Several broad peaks are observed in the UV data and alcohols 12 and 13 had to be extracted from the mass spectrometer signal to be visible. The amine chromatogram is self explanatory in the fact that this class of compounds is too strongly retained.
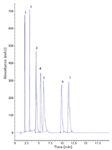
Figure 8: Commercially available amines analysed under standard chromatographic conditions on the nonendcapped Basic column according to the standard chromatographic conditions used in this study.
Conclusion
A library of test analytes with a broad range of molecular properties was used to characterize the suitability of several investigational stationary phases as general tools for SFC separation. Although no single column was identified with broad utility across the entire set of compounds, amides generally produced the most consistent peak shape across all stationary phases evaluated and amines tended to have the worst peak shape. The amines were also slightly less retentive, but frequently yielded inefficient peak shapes with severe tailing (for example, in the case of silica). Carboxylic acids were on average the most strongly retained class of compounds on most of the columns evaluated. An estimated, normalized retention order would be carboxylic acids < amines < alcohols < amides with the carboxylic acids being the most strongly retained. From the endcapping study, it appears the silanol groups on the nonendcapped stationary phases play a role in maintaining the quality of separations and demonstrates that silanols do play a significant role in the separation mechanisms in SFC.

Figure 9: Example of a single phase not being universal. All four data files were generated using standard chromatographic conditions on the nonendcapped nitro column. Top: amide mix; second from top: carboxylic acid mix; third from top: alcohol mix; and bottom: amines.
We developed the colour-coded chart shown in Figure 10 to summarize our findings. This chart is based upon the four chemical classes and the columns studied. In the chart, we identified the "best" column for each chemical class by taking into account selectivity, retention and peak shape. These "best" columns can be used as a first line for separating chemical in the four chemical classes:
- Ethyl pyridine (nonendcapped) for carboxylic acids
- DEAP (nonendcapped) for alcohols
- Nitro phenyl (nonendcapped) for amides
- GreenSep Basic (nonendcapped) for amines

Figure 10: Summary table of recommended stationary phases based on analyte structural classes.
In the future, more chemical classes of compounds will be subjected to these stationary phases to enable matching of additional chemical classes with its appropriate stationary phase. Ultimately, the approach described in this study will lead to the development of new stationary phases for SFC and may lead to the development of a universal SFC stationary phase.
Acknowledgments
The authors would like to acknowledge Bill Shipe, Scott Harrison, Dave Kohler, Rowena Ruzek, Brad Feuston, Eden McQueen and Chris Welch for their help with this project.
Matt Przybyciel is vice-president and technical director for ES Industries in West Berlin, New Jersey, USA. He directs research, development and manufacturing of HPLC, SFC and chiral column products.
Ray McClain is a senior research associate at Merck & Company's West Point Pennsylvania Facility, USA. Ray manages the analytical and preparative chromatography efforts in the Department of Medicinal Chemistry.
"Column Watch" Editor Ronald E. Majors is a senior scientist at the Columns and Supplies Division, Agilent Technologies, Wilmington, Delaware, USA and is a member of LCGC Asia Pacific's editorial advisory board. Direct correspondence about this column should be addressed to "Column Watch", LCGC Asia Pacific, 4A Bridgegate Pavilion, Chester Business Park, Wrexham Road, Chester, CH4 9QH, UK, or e-mail the editor, Alasdair Matheson, at amatheson@advanstar.com
References
(1) H. Bui, T. Masquelin, T. Perun, T. Castle, J. Dage and M-S. Kuo, J. Chromatogr. A. 1206, 186 (2008).
(2) J. Lundgren, J. Salomonsson, O. Gyllenhaal and E. Johansson, J. Chromatogr. A. 1154, 360 (2007).
(3) C. White and J. Burnett, J. Chromatogr. A. 1074, 175 (2005).
(4) B. Bola, M. Greig, M. Ventura, W. Farrell, C.M. Aurigemma, H. Li, T.L. Quenzer, K. Tivel, J.M.R. Bylund, P. Tran, C. Pham and D. Phillipson, Int. J. Mass Spectrom 238, 85 (2004).
(5) R.A. Coe, J.O. Rathe and J.W Lee, J. Pharma. Biomed. Anal. 42, 573 (2006).
(6) I. Brondz, D. Ekeberg, D.S. Bell, A.R. Annino, J. Arild Hustad, R. Svendsen, V. Vlachos, P. Oakley, G.J. Langley, T. Mohini, C-G. Amaury and F. Mikhalitsyn, J. Pharm. Biomed. Anal. 43, 937 (2007).
(7) M. Maftouh, C. Granier-Loyaux, E. Cavana, J. Marini, A. Pradines, Y. Vander Heyden and C. Picard, J. Chromatogr. A. 1088, 67 (2005).
(8) Y. Zhang, W. Watts, L. Nogle and O. McConell, J. Chromatogr. A. 1049, 75 (2004).
(9) L. Miller and M. Potter, J. Chromatogr. B. 875, 230 (2008).
(10) Z. Pirzada, M. Personick, M. Biba, X. Gong, L. Zhou, W. Schafer, C. Roussel and C.J. Welch, J. Chromatogr. A. 1217, 1134 (2010).
(11) D. Mangelings and Y. Vander Heyden, J. Sep. Sci. 31, 1252 (2008).
(12) C. Welch, W. Leonard, J. DaSilva, M. Biba, J. Albaneze-Walker, D. Henderson, B. Laing and D. Mathre, LCGC N. Amer. 23(1), 16, (2005).
(13) L.T. Taylor and M. Ashraf-Khorassani, LCGC N. Amer. 28(9), 810 (2010).
(14) T. Berger and B. Berger, LCGC N. Amer. 28(5), 344 (2010).
(15) C. West and E. Lesellier, J. Chromatogr. A. 1110, 191 (2006).
(16) C. West and E. Lesellier, J. Chromatogr. A. 1110, 200 (2006).
(17) C. West and E. Lesellier, J. Chromatogr. A. 1203, 105 (2008).
(18) C. West and E. Lesellier, J. Chromatogr. A. 1169, 205 (2007).
(19) E. Lesellier, J. Chromatogr. A. 1216, 1881 (2009).
(20) J. Isbell, J. Comb. Chem. 10, 150 (2008).
(21) W. Shipe, F. Yang, Z. Zhao, S. Wolkenberg, M. Nolt and C. Lindsley, Heterocycles 70(1), 655 (2006).
(22) X. Zhang, M. Towle, C. Felice, J. Flament and W. Goetzinger, J. Comb. Chem. 8, 705 (2006).
(23) R. McClain, A. Dudkina, J. Barrow, G. Hartman and C. Welch, J. Liquid Chrom. & Related Techn. 32(4), 483 (2009).
(24) M. Ventura, W. Farrell, C. Aurigemma, K. Tivel, M. Greig, J. Wheatley, A. Yanovsky, K. Milgram, D. Dalesandro, R. DeGuzman, P. Tran, L. Nguyen, L. Chung, O. Gron and C. Koch, J. Chromatogr. A. 1036, 7 (2004).
(25) T. Berger, K. Fogleman, T. Staats, P. Bente, I. Crocket, W. Farrell and M. Osonubi, J. Biochm Biophys Methods 43, 87 (2000).
(26) J. Hert, P. Willett, D.J. Wilton, P. Acklin, K. Azzaoui, E. Jacoby and A. Schuffenhauer, Org. Biomol. Chem. 2, 3256 (2004).
(27) C. West and E. Lesellier, J. Chromatogr. A. 1110, 181 (2006).
(28) J. Blackwell, R. Stringham and J. Weckwerch, Anal. Chem. 69, 409 (1997).

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Quantifying Terpenes in Hydrodistilled Cannabis sativa Essential Oil with GC-MS
April 21st 2025A recent study conducted at the University of Georgia, (Athens, Georgia) presented a validated method for quantifying 18 terpenes in Cannabis sativa essential oil, extracted via hydrodistillation. The method, utilizing gas chromatography–mass spectrometry (GC–MS) with selected ion monitoring (SIM), includes using internal standards (n-tridecane and octadecane) for accurate analysis, with key validation parameters—such as specificity, accuracy, precision, and detection limits—thoroughly assessed. LCGC International spoke to Noelle Joy of the University of Georgia, corresponding author of this paper discussing the method, about its creation and benefits it offers the analytical community.











