Syringes for Gas Chromatography
LCGC Europe
Most modern GC autosamplers employ high-speed actions by default.
In liquid chromatography (LC), the syringe functions primarily as a pipette or liquid-transfer device that loads a sample loop. The syringe generally does not take an active role in injection, which occurs only after sample has been displaced from the syringe. The same is true of most gas chromatography (GC) gas-sampling valves — the time at which the gaseous sample is injected into the column is separate from the moment that it is transferred into the injection system. In GC analysis of liquid samples, however, the syringe becomes an integral part of the inlet during injection: sample, in liquid or gaseous form, starts to enter the column as soon as the syringe enters the inlet.
In GC inlet systems for liquids, the injection technique, choice of syringe and inlet operating conditions all play a crucial role in the injection process. Two principal sample-transfer mechanisms move sample from the syringe into the inlet while the syringe is in the inlet. First, liquid-sample transfer takes place as the syringe plunger is depressed and liquid is expelled from the syringe tip. In cold injection, where the inlet temperature is not high enough to produce significant solvent vaporization, this pipette-like action is the only major sample-transfer mechanism. However, a competing process occurs in a hot injector. Within a few tenths of a second after the needle enters a hot inlet, sample begins to evaporate inside the needle. Bubble formation and concomitant increased internal pressures force some liquid out along with the vapour, so that part or all of the sample contained in the syringe needle volume is introduced into the inlet as a mixture of liquid and vapour. As the plunger is depressed, additional room-temperature liquid sample is forced from the syringe through the needle, which cools the needle and suppresses but does not entirely stop in-needle evaporation. The needle heats up again once the syringe plunger motion ceases, which causes additional sample vaporization from the needle into the inlet. All of these processes take place in a matter of seconds. The total amount of sample that is actually injected into the inlet depends strongly upon these two processes, their timing, the volumes involved and the inlet conditions. Along with judicious injection condition control, a good understanding of the role of the syringe in these processes will help gas chromatographers obtain better injections.
Sample Distortion During Injection
Syringe-needle effects influence not only the injected sample volume; they can also modify the relative amounts of individual sample components that enter the inlet. To understand this secondary effect, consider that not all sample components have the same vapour pressures at a given temperature. Lower molecular weight compounds have higher vapour pressures, and conversely, heavier molecules have lower vapour pressures. This differentiation forms the basis for simple thermal fractionation of a mixture of compounds — in a distillation column, for example.
Unfortunately, for many gas chromatographers, the same kind of thermal fractionation process can occur in the syringe needle during injection. When in-needle vaporization occurs, the lighter components vaporize first and leave the syringe needle quickly. Heavier compounds take longer to evaporate and leave the syringe needle more slowly. The effect of initial needle heating during injection is remediated largely by subsequent bulk liquid transfer through the needle: straggling heavy compounds will be rinsed out. At the end of injection, however, if the needle is withdrawn from the inlet before complete transfer of all compounds can occur, then the sample that enters the inlet system will contain more of the volatile components and less of the heavier components than were present in the sample before injection. This effect is called "mass discrimination" because it tilts the sample composition according to components' vapour pressures, which are related largely to their masses.
Two other thermal side-effects go hand-in-hand with needle fractionation. Many compounds are sensitive to thermal stress, especially in the presence of a hot steel syringe needle, and decompose rapidly when subjected to high inlet temperatures: high molecular weight glycerols are one good example. Polar compounds can become strongly adsorbed on the needle surface. Many of the polar polyaromatic hydrocarbons (PAHs) as well as a host of chlorinated pesticides suffer from this problem. In both instances, the use of nickel syringe needles, or needles that have been deactivated by advanced surface treatments, can help tremendously, as can deactivation of inlet liners and injector inner surfaces exposed to the sample. In these situations, thermally mild injection techniques such as cold on-column injection or programmed temperature vaporization usually produce results superior to what can be obtained by careful deactivation of needles and other hot injection materials, but the specialized inlets needed for these somewhat more complex techniques might not be available.
Judicious data-system calibration and the use of multiple internal standards can compensate for inaccuracies caused by mass discrimination, decomposition and adsorption, as well as for a host of other injection problems. However, the precision and repeatability of multiple analyses are affected adversely when the relative amounts of individual components strongly depend upon injection conditions. Heavier components — against which injection strongly discriminates — or any other components that do not come through the injection process at 100%, will exhibit degraded lower detection limits (LDLs) as well. Degradation of precision, repeatability and LDL are significant effects even for samples that do not span a wide range of volatilities and do not suffer from mass discrimination per se.
When this type of injection problem does arise, its effects must be either suppressed or controlled by applying special syringe-handling techniques or by using cold injection if possible. The "sandwich" technique is a good example. The syringe is first filled with 1 μL or so of pure liquid solvent followed by a small air plug. The sample is pulled into the syringe, then another plug of air, and finally some more solvent. The entire liquid array is pulled up into the syringe so that the needle is largely empty. Upon injection, only a little solvent is in the needle as it enters the inlet. Sample passes through the needle as the syringe plunger is depressed, followed by a plug of solvent that forces any remaining sample into the inlet. Only pure solvent is left in the needle at the end of injection, so needle fractionation does not occur. The drawback of the sandwich technique is that it significantly increases the amount of solvent that enters the inlet, which might overload the inlet with solvent vapour or broaden the solvent peak and interfere with early-eluted analytes.
A high-speed autosampler can also remediate needle fractionation. By injecting the sample in a very short time, typically less than 500 ms and certainly much faster than a human can achieve, the syringe needle residence time in the inlet is insufficient for significant needle heating to occur, and so needle fractionation is eliminated. Another benefit of a fast injection is the suppression of sensitive-component thermal decomposition or adsorption on hot steel needle surfaces, again as a result of reduced contact times. Most modern GC autosamplers employ high-speed actions by default.
In-needle fractionation and other thermal effects are some of the syringe-related processes that interfere with injection accuracy and repeatability. There are many other effects that occur before, during and after sample has entered the inlet system: see the book by Grob for a comprehensive discussion.1
Types of Syringes
The choice of syringe type plays an important role in obtaining the best performance from a gas chromatograph. There are two basic kinds of manual syringes: plunger-in-barrel [Figure 1(a)] and plunger-in-needle [Figure 1(b)]. In the first type, sample is drawn up into a calibrated glass barrel; in the second, the entire sample is contained in the needle, and a thin wire plunger fitted into the bore of the needle forces liquid out during injection.
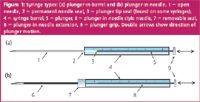
Figure 1
Plunger-in-Barrel Syringes
In this type of syringe, sample is contained in the glass barrel [see Figure 1(a)]. Since you can see the sample, it is easy to observe the presence of liquid sample and determine if the syringe needle is obstructed. In use, the syringe is usually overfilled with liquid and the extra is then ejected. A final clinging droplet can be removed from the needle tip with a clean laboratory wipe or by rinsing with solvent. To gauge the injected volume, consider that the amount displaced from the markings on the syringe barrel does not include sample vaporized from the needle and is only an approximation. The actual amount injected is closer to the needle volume (about 0.7 μL for a 10 μL syringe) plus the amount displaced. Another way to determine injected volume is to load the syringe with sample, pull the plunger back and read the total volume of the liquid plug. After injection, pull the plunger back slightly, read the remaining liquid volume, and subtract it from the original amount. In this way, sample evaporated from the needle is included in the total amount. Plunger-in-barrel syringes can be used for most routine applications. The common 10 μL syringe can reliably inject liquid volumes ranging from 1 μL to 10 μL.
Plunger-in-barrel syringes can be cleaned in several ways. Pumping solvent in and out of the syringe is effective and can be a good idea between injections to remove leftover sample before it evaporates and leaves a film of residue behind. Eventually, a layer of nonvolatile residue and some particles will contaminate a syringe. The slightest amount of particulate matter inside the syringe barrel will immediately begin to degrade the precision fit between the plunger and the inner glass barrel bore, so cleanliness is crucial for the longest syringe life. This is even more important for plunger-in-barrel autosampler syringes, in which the plunger is cycled up and down many more times than for manual injection. For best results with slightly contaminated samples, use a syringe that has a polymer seal on the plunger tip.
A commercial syringe cleaner heats the syringe needle to as high as or above the injection temperatures under a mild vacuum. This will remove some residual material, but it will not remove nonvolatile residues and particulates, nor will it clean the barrel and plunger. In most syringes of this type, the plunger is removed easily for solvent washing. With the plunger removed, the solvent can be drawn through the syringe body from the back with mild suction. Be careful, however, that particles inside the upper end of the barrel are not drawn into the needle where they could block flow. If the needle does become blocked, insert a fine cleaning wire — usually supplied with the needle — to remove the blockage. Whatever cleaning method you use, make sure you do not use abrasives because they will destroy the precision fit between.
Plunger-in-Needle Syringes
The plunger-in-needle syringe is useful for injection volumes < 5.0 μL; the 1.0 and 0.5 μL sizes are more common. In this type of syringe, the needle contains the entire sample [see Figure 1(b)]. Sample-distortion effects that are as a result of partial sample displacement are reduced because the plunger extends the length of the needle, but these effects are not eliminated entirely. The injected amount can be read directly off the barrel. But these syringes are more complex in construction, with a removable needle and seal assembly. You cannot see liquid sample in the needle, so it is difficult to tell if the needle is obstructed.
Cleaning plunger-in-needle syringes is more difficult because the plunger should not be removed except for major repairs (such as replacing the needle). Nevertheless, you can get good results by pumping solvent into the syringe to remove soluble residue, followed by heating the needle under vacuum. Note that for any GC syringe, only the needle should be heated above 50 °C. Plungers can stick, glass barrels can crack and syringe seals can develop leaks if the entire assembly is overheated.
Needle Styles
Needles come in fixed and removable styles, with various tip shapes. The fixed-needle syringes are the most economical, but if the needle is damaged, the syringe cannot be repaired. Removable-needle syringes will last longer, but must be maintained by checking that the needle-to-barrel seal remains intact as the moving parts wear. Never use a Luer-lock (medical) type of removable syringe needle for GC liquid injections because these have an excessively large dead volume. Instead, select one of the types specifically designed for GC and designated as a "microsyringe" with a zero-dead-volume seal.
Needle tip shape can affect septum life. Most manufacturers produce a standard tip with a 20° bevel that is optimized for penetrating GC septa. The 14° medical bevel is not recommended. Plunger-in-needle syringes have blunt, nonbevelled tips. Of these, the dome-shaped and the electropolished taper types are the best for the septum. The correct needle tip minimizes septum coring and the deposition of septum pieces inside the inlet, which otherwise can produce "ghost" peaks. In some situations, a needle with a side hole can be advantageous.
Syringe needles also come in a variety of dimensions. An outer diameter of 0.47 mm (26 gauge) is fairly standard and permits the needle to penetrate a 0.53 mm i.d. wide-bore fused-silica column in an on-column style inlet. The somewhat stronger 0.7 mm (22 gauge) o.d. needle is often used if bent needles are a problem. However, this larger needle can shorten septum life. Syringe needle lengths are generally either 5 or 7 cm. The length required is usually a function of the inlet design, and the manufacturer's recommendations should be followed.
Specialized Syringes
Several types of specialized syringes are available. The most familiar is the autosampler syringe. Original equipment manufacturer supplied syringes are best, but compatible syringes are available from syringe manufacturers that might have some features not otherwise available, such as specific needle tips or plunger seals.
There are two types of autosampler syringes: front-loading and flow-through. The front-loading type, found on essentially all modern GC autosamplers, mimics a human operator's motions (except much more quickly and precisely). With the flow-through type, found on older autosamplers, sample is pushed from a pressurized sample vial through the syringe and out to a waste receptacle. Once the autosampler is loaded with sample, injection occurs in the normal fashion. These syringes tend to be more tightly integrated with the autosampler mechanics and are harder to replace. As mentioned previously, and worth repeating, is the fact that autosampler syringes need better care than manual syringes because they are subjected to greater mechanical stresses and more operating cycles.
Another familiar speciality syringe is the cold on-column syringe. This is generally a plunger-in-barrel type with a special removable needle made of narrow-bore steel or fused-silica tubing. The needle is designed to penetrate a narrow-bore (0.20 or 0.25 mm i.d.) capillary column to accomplish on-column injection. Such syringes are not interchangeable across different injector designs; the manufacturer's specifications must be followed. On-column syringes can be more susceptible to needle blockage because of their smaller inner diameters. Many on-column injectors use a precolumn or retention gap made of uncoated 0.53 mm i.d. tubing, which accepts a standard 26 gauge syringe needle.
Syringe Repair
Many syringe manufacturers offer syringe repair kits, needle replacements and repair service. Most removable needles can be replaced in the field if the rest of the syringe is in good shape. For plunger-in-needle syringes, needle damage is often accompanied by plunger damage, and in such instances, it is necessary to replace both as a matched pair.
Syringe plungers are generally not field-replaceable (or interchangeable) because of the tight matching tolerances between plunger and barrel. One exception is microlitre gas-tight syringes with PTFE-tipped plungers, which will accept replacement plungers if the barrel is not otherwise damaged.
Is syringe repair worth the cost? In the case of plunger-in-needle syringes, the answer is a definite "yes". Repair kits of matched plungers and needles are a quarter to a third of the cost of a new syringe. For the less-expensive plunger-in-barrel types, replacement needles are as much as half the cost of a new unit, and instead it might be worthwhile to attempt to reduce syringe damage and extend syringe life through better handling and cleaning procedures.
Syringe Accessories
A few syringe and sampling accessories are especially useful. First, a syringe rack is a good way to keep syringes in one place, rather than on top of the gas chromatograph or the bench, where they can be pushed off and broken easily. Syringe labels are also handy to keep track of which syringe is used for which type of sample.
Another useful item is one of the various repeating adapters that permit the plunger to be returned to the same position each time, which can improve the repeatability of multiple injections. Also, repeating adapters act as guides and prevent plunger bending or accidental withdrawal from the barrel, a feature that is important on plunger-in-needle syringes. Several manufacturers also make syringes with extended or reinforced barrels and plungers that prevent damage from a heavy hand.
Conclusion
When viewed as an active part of an inlet, the syringe takes on new importance. While injection technique is crucial, the proper care and cleaning of the syringe can contribute significantly to improved accuracy and precision. Choosing the correct syringe to meet sample and injector requirements, and then applying and maintaining it correctly, can go a long way toward producing better and more reliable analytical results.
"GC Connections" editor John V. Hinshaw is senior staff engineer at Serveron Corp., Hillsboro, Oregon, USA and a member of the Editorial Advisory Board of LCGC Europe. Direct correspondence about this column to "GC Connections", LCGC Europe, Advanstar House, Park West, Sealand Road, Chester CH1 4RN, UK, e-mail: dhills@advanstar.com
For an ongoing discussion of GC issues with John Hinshaw and other chromatographers, visit the Chromatography Forum discussion group at http://www.chromforum.com
References
1. K. Grob, Split and Splitless Injection for Quantitative Gas Chromatography: Concepts, Processes, Practical Guidelines, Sources of Error, 4th, Completely Revised Edition (Wiley, Hoboken, New Jersey, USA 2001).
Study Examines Impact of Zwitterionic Liquid Structures on Volatile Carboxylic Acid Separation in GC
March 28th 2025Iowa State University researchers evaluated imidazolium-based ZILs with sulfonate and triflimide anions to understand the influence of ZILs’ chemical structures on polar analyte separation.
Quantifying Microplastics in Meconium Samples Using Pyrolysis–GC-MS
March 26th 2025Using pyrolysis-gas chromatography and mass spectrometry, scientists from Fudan University and the Putuo District Center for Disease Control and Prevention detected and quantified microplastics in newborn stool samples.













