Simultaneous Determination of Chelating Agents by Ion-Suppression and Ion-Pair Chromatography in Wastewater
LCGC Europe
Chelating agents such as NTA, CDTA and DTPA are considered by ANDRA (the French national agency for radioactive waste management) as compounds to be investigated because they may enhance the release of radioactive isotopes in the environment.
This article describes two methods for analysing chelating agents found in nuclear waste:
- ion-suppression chromatography using an anion exchange stationary phase and mobile phase consisting of a nitric acid solution and pure water gradient. UV detection was performed at 330 nm after the reaction with a postcolumn reagent composed of iron nitrate in perchloric acid.
- ion-pair chromatography with a mobile phase consisting of a mixture of nitric acid, tetrabutylammonium hydrogenosulphate, tetrabutylammonium hydroxide and iron chloride. A reversed-phase material was used as a stationary phase and detection was performed by direct measurement of the UV absorption at 260 nm.
The quantification limits were lower for ion-pair chromatography than for ion-suppression chromatography.
Both methods were easy to implement and allow a multi-element separation in less than 30 min with low detection limits.
Nuclear waste regulations are becoming increasingly stringent and aminopolycarboxylic acids must be analysed in nuclear waste packages. Indeed, these species form water-soluble complexes with most heavy metals, which may result in the contamination of soils by radioactive elements. For example, studies at Oak Ridge National Laboratory (Tennessee, USA) have demonstrated that ethylenediaminetetracetic acid (EDTA) caused the low-level migration of 60Co from intermediate-level liquid waste disposal pits and trenches.1
Thus, chelating agents such as nitrilotriacetic acid (NTA), ethylenediaminetetracetic acid, cyclohexanediaminetetracetic acid (CDTA) and diethylenetriaminepentacetic acid (DTPA) are considered by ANDRA (the French national agency for radioactive waste management) as compounds to be investigated because they may enhance the release of radioactive isotopes in the environment.2
Ringbom explained that the chelating agents exhibit strong complexing power because of their carboxylic functions and their nitrogen and oxygen atoms.3 The complexation constants of iron (III) with chelates shows that these complexes are particularly stable.4
Buchberger and Mülleder demonstrated that the stability of the complexes depends on the pH.5 When the pH increases, the chelating agents are more and more deprotonated and exhibit their complexing power (but obviously, at increasing pH metal ions may precipitate, which decreases the apparent stability).
The chelating agents, such as EDTA, are largely used for the detection of metals by UV detection. For this reason metal (iron) complexes are generally used to perform the detection of these aminopolycarboxylic acids. Different analytical techniques have been used to perform the determination of chelating agents.
Owens et al. studied EDTA and NTA with different metallic ions by capillary electrophoresis (CE), the determination limit for the iron–EDTA complex was 4 mg/L.6 Padarauskas et al. also separated EDTA, CDTA and DTPA by CE as complexes with metallic ions.7 The determination limits for the complex iron–DTPA was 0.9 mg/L. Laamanen et al., determined DTPA, EDTA and NTA by CE after complexation with Cu ions.8
Collins et al. exploited ion chromatography/mass spectrometry to quantify metal–EDTA complexes in soil solution; with this method it was possible to identify and quantify the metal–EDTA complexes in environmental samples.9 Nevertheless, iron (III)–EDTA, Ca–EDTA, Mg–EDTA and EDTA itself were not detected. Miller et al. analysed EDTA in dried bloodstains by ion chromatography and electrospray liquid chromatography/mass spectrometry/mass spectrometry (LC–MS–MS).10 Nevertheless, the determination limit was only 5 mg/L. Dodi et al. used electrospray LC–MS to investigate EDTA traces in nuclear wastes. Quantification limits of 2 μg/L (for 20 μL injected) were achieved.11
A German standard, dealing with the analysis of water, wastewater and sludge, describes the determination of NTA, EDTA and DTPA through LC; determination limits were 70 μg/L, 40 μg/L and 120 μg/L, respectively.12 A similar work was published as an application note from Macherey Nagel to investigate EDTA, NTA, DTPA.13
A French standard was published for the determination of chelating agents (EDTA, HEDTA and DTPA) in fertilizers using ion chromatography.14 Moreover, an application note from Dionex proposed the quantification of EDTA, NTA and phosphates by ion-suppression chromatography.15
The determination of these chelating agents by gas chromatography has also been reported1,16,17 but the main drawback of this technique is the need to perform a derivatization step prior to injection. All samples must be treated with an esterification reagent prior to analysis but the yield of the reaction may depend on the sample matrix.
Nowack and co-authors have reported works in ion chromatography coupled with ICP/MS to separate organometallic complexes with a mobile phase composed of NH4NO3.18 The quantification limit for EDTA is between 0.5 and 1.0 μM.
The aim of this article is to propose and to compare two ion chromatographic methods (ion-suppression and ion-pair chromatography) for the simultaneous determination of four aminopolycarboxylic compounds: NTA, EDTA, CDTA, DTPA (Figure 1) with low detection limits.
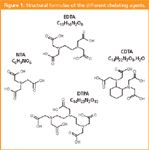
Figure 1
Experimental
Materials: For all eluent and standard preparations, deionized (DI) water was provided by a point-of-use water-purification system from Millipore (St Quentin en Yvelines, France). Milli-Q gradient A10 was linked to an Elix and Rios system. The eluents and DI water reservoirs were purged with helium before performing the separation. The standard solutions were prepared from EDTA (Titriplex III), NTA (Titriplex I), CDTA (Titriplex IV) and DTPA (Titriplex V) purchased from Merck (Darmstadt, Germany).
Ion-suppression chromatography: A nitric acid solution (100 mM) was prepared from a 69% solution purchased from Merck. This solution was poured into a 1 L flask and a second flask was filled with DI water. The postcolumn reagent was prepared in a 1 L flask with 1 g of iron (III) nitrate nonahydrate purchased from Prolabo (Paris, France) and 20 mL of 70–72% perchloric acid solution from Merck.
Ion-pair chromatography: Preparation of the eluent required a diluted nitric acid solution (prepared from a commercial 69% solution purchased from Merck), tetra-n-butylammonium hydroxide (prepared from a commercial 40% solution) from VWR (Fontenay sous bois, France), tetra-n-butylammonium hydrogenosulphate from Merck and anhydrous iron (III) chloride from VWR.
Apparatus and Columns
On both systems, a Dionex (Sunnyvale, California, USA) DX 500 ion chromatograph equipped with a manual degassing system, an automatic sampler, a quaternary gradient pump and an AD 20 absorbance detector was used.
The sample loop size was 500 μL on both methods. Samples were introduced into the instrument via an AS40 automated sampler, using 5 mL PolyVials with pain caps. All instrument modules were purchased from Dionex. Instrument control and data collection were performed with a PC and Peaknet software (Dionex).
For ion-suppression chromatography an AS7 column (4 × 250 mm) equipped with an AG7 guard column (4 × 50 mm from Dionex) was implemented. The mean particle size of the resin was 10 μm (data given by the manufacturer). A GP 40 gradient pump mixed the eluent constituents (water and 100 mM HNO3) for the gradient program used. The flow-rate was adjusted to 1.0 mL/min, and the UV/visible module was set at a wavelength of 330 nm. With this system, a postcolumn reagent was mixed with the mobile phase through a mixture chamber, just before detection.
In ion-pair chromatography the stationary phase tested was an RP Nucleodur C18 Pyramid (4 × 250 mm) from Macherey Nagel (5 μm mean particle diameter). The GP 40 gradient pump was also used but no gradient program was established. The flow-rate was adjusted during the separation.
The flow-rate was 0.8 mL/min for 15 min, after which it was set at 1.10 mL/min to reduce the elution time of the last peak (CDTA). Detection was performed with an absorbance detector (AD 20) at 260 nm.
Standard Preparation
Eppendorf (Hamburg, Germany) research pro pipettes were used to prepare standard and sample solutions. A stock solution (500 mg/L) of NTA, EDTA, DTPA and (100 mg/L) CDTA was prepared by dissolving the commercially available product in deionized water. Diluted solutions for analysis were freshly prepared everyday of analysis.
On Guard M Resin
This ion exchanger (purchased from Dionex) permits the retention of cationic metals and was used to perform the tests dealing with the influence of metals.
Calculations
Statistical calculations were performed using the formula given below (from reference 19):
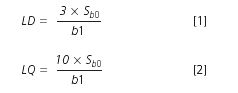
where Sb0 is the standard deviation measured with a low level standard and b1 is the slope of the calibration curve.
Results and Discussion
Ion-suppression chromatography: Usually, chromatography of anions is performed with a basic mobile phase. In the instance of aminopolycarboxylic compounds, these anions would be too strongly retained on the resin with a basic media; for this reason it was necessary to proceed by ion-suppression chromatography with an acidic mobile phase.
The eluent was composed of a gradient between water and a 100 mM nitric acid solution ([HNO3] = 15 mM from 0–7 min, to reach 20 mM at 17 min). The flow-rate was adjusted to 1 mL/min and the observed pressure was about 7.6 MPa (1100 psi). The chromatogram displayed in Figure 2 shows that the first eluted compound is CDTA followed by EDTA, then DTPA and NTA.
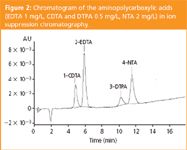
Figure 2
This order of elution may be explained if we consider the pKa1 of the chelating agents (Table 1). The pH of the mobile phase was 2.6; subsequently, the higher the pKa1, the lower the concentration of the deprotonated form (A- ) and thus the species is less retained on the resin. The pKa1 values (and the [A- ]/[AH] calculated ratio) indicated in Table 1 allow us to explain the observed elution order.
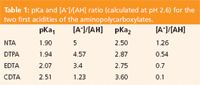
Table 1: pKa and [A-]/[AH] ratio (calculated at pH 2.6) for the two first acidities of the aminopolycarboxylates.
Linearity was obtained up to 1 mg/L, according to the XP T 90–210 AFNOR standard. The least-squares equations and the correlation coefficients for each compound are reported in Table 2.

Table 2: Calibration curves parameters, detection limits and quantification limits calculated for the ion suppression chromatography method.
Then the limits of detection (LD) and quantification (LQ) were calculated with the formula given earlier, according to XP T 90–210 AFNOR standard; the results are shown in Table 2. The mean noise observed with this method was 65 μAU.
Ion-pair chromatography: The mobile phase was composed of 0.6 mM HNO3, 2.6 mM tetrabutylammonium hydroxide, 7.53 mM tetrabutylammonium hydrogenosulphate and 37 μM anhydrous iron (III) chloride (from reference 13). The pH of this mobile phase was 2.33.
The tetrabutylammonium groups of the ion pairing reagents are maintained close to the C18 alkyl chains by hydrophobic interaction and the cationic ammonium moiety allows the reverse phase to have a similar behaviour as a dynamic anion exchanger.
The flow-rate was adjusted to 0.8 mL/min and the observed pressure was 11 MPa (1600 psi); when the flow-rate was increased at 1.1 mL/min, the pressure reached values of approximately 17.6 MPa (2550 psi).
As we can see in Figure 3, the elution order is different from the one observed for ion-suppression chromatography. In fact, the retention time seems to be correlated with the number of carboxylic functions present in the compound; the more the chelating agent has carboxylic functions, the more the ferric complex is retained on the stationary phase.
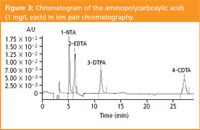
Figure 3
We must note that CDTA constitutes an exception because this molecule exhibits a hydrophobic structure (C6 cycle) (Figure 1) and thus, strong hydrophobic interactions occur between CDTA and the alkyl groups of the stationary phase.
Linearity was observed up to 500 μg/L. The least-squares equations and the correlation coefficients for each compound are presented in Table 3.

Table 3: Calibration curves parameters, detection limits and quantification limits calculated for the ion pair chromatography method.
The detection and determination limits are also shown in Table 3. They were calculated in the same way as for ion-suppression chromatography. The noise observed with this method was 37 μAU (half that observed with ion-suppression chromatography).
Comparison of the two methods: Ion-pair chromatography induced lower detection limits than ion suppression chromatography (for the same injected volume).
We noted that the background signal was higher (65 μAU) in ion-suppression than in ion-pair chromatography (37 μAU). This may be explained by the technique itself.
With ion-suppression chromatography, a postcolumn reagent was added to the flow issued from the column through a mixture chamber (thin tube containing micro balls). This operation constitutes an important source of analytical noise and for this reason, among others, the technique is less sensitive than ion-pair chromatography, which does not need a postcolumn reagent; iron (III) being present in the mobile phase. We might introduce iron (III) in the mobile phase with the ion-suppression chromatography method, but this could be detrimental for the anion exchange resin.
As far as the elution time is concerned, we observe that the ion suppression method allowed a faster separation than ion pair chromatography in spite of a flow-rate modification performed at 15 min after the start of the run (increase of the flow-rate from 0.8–1.1 mL/min) for this last method.
However, we preferred to implement ion-pair chromatography, which exhibits lower detection limits. (The possibility of having an automatic sampler connected to the chromatograph allowed us to accept relatively long retention times.)
Influence of metals (ion pair chromatography): Usually the investigated chelating agents are present in media, which may contain metals and thus, organometallic complexes can be formed because of the presence of these metals in the sample. For that reason, tests were performed with different metals previously added in a synthetic solution of the aminopolycarboxylic compound. The tested metals were copper, cobalt and lead. We prepared aqueous solutions with a 103 ratio metal/chelating agent.
In this instance, a complex was formed between the metal and the chelating agent but a metal excess remained in the solution. The samples were investigated by ion pair chromatography and the results are reported in Figure 4. We may observe that cobalt modified the concentration of NTA and CDTA, copper modified the concentration of CDTA and DTPA and lead did not alter any concentration.
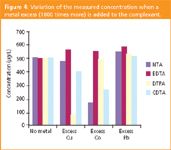
Figure 4
To remove metals present in the sample, we pretreated an aliquot of the sample with a resin adapted for metal retention (On Guard M resins from Dionex). For that purpose, an aliquot was previously eluted through the resin cartridge for removing the metal. The results reported in Figure 5 show that after this pretreatment, all the metal interferences were removed except the cobalt CDTA interference which remains significant.
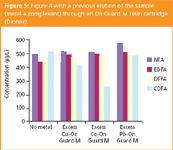
Figure 5
Therefore, in the situation of a matrix containing a high metallic charge, the On guard M resin may be used to limit the influence of metals but it does not completely suppress the effect of all the metals. We can note that the pKc value of cobalt with CDTA is particularly high (21.9).
Application to a real sample: A real sample, containing a high load in salt (NaCl and Na2SO4 at about 10 g/L), in which we had to measure EDTA, was investigated by ion-pair chromatography. The result is presented in Figure 6. Standard addition allowed us to prove that the main peak was EDTA. The measurement was then performed by ion-pair chromatography (concentration found = 29.4 mg/L) and by a well-known method, LC/ESI/MS (value found = 30.7 mg/L).11 The high saline load did not perturb the measurement and so ion-pair chromatography may be implemented for such samples.
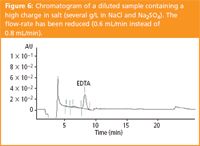
Figure 6
Conclusion
The environment is an important preoccupation and for this reason chelating agents must be taken into account in the management of (nuclear) waste because they can induce water-soluble complexes with most heavy metals. The two methods we developed are easy to implement and permit the simultaneous analysis of NTA, EDTA, DTPA and CDTA in less than 30 min.
Ion-suppression chromatography allows the separation of NTA, EDTA, CDTA and DTPA in less than 20 min and the quantification limits are in the range of 0.18–0.45 μmole/L (with a 500 μL injection loop).
The ion-pair chromatography method is most interesting because it is more sensitive. This is probably because the technique does not require the addition of a postcolumn reagent (this reagent being present in the mobile phase) via a mixture chamber which is one of the main causes of analytical noise. With this latter technique, the detection limits are in the range of 0.04–0.1 μmole/L (with a 500 μL injection loop) but the last peak (CDTA) is eluted at 28 min. This relatively long separation time does not constitute a problem if an automatic sampler is connected to the chromatograph.
We have shown that transition metals could influence the signal if they are present at high concentrations in the sample. Therefore, the previous elution of an aliquot of the sample through an On Guard M resin cartridge enables this effect to be reduced.
Acknowledgements
We acknowledge Professor A.M. Siouffi for fruitful discussion.
Alain Dodi graduated from the High School of Chemistry of Lyon (ESCIL) France (1989) and possesses Masters degrees in both organic chemistry (Marseille, France, 1987) and analytical chemistry (Lyon, France, 1989). He began to work at the French agency for atomic energy (C.E.A.) as a researcher in organic synthesis from 1990 to 1993 and since then in analytical chemistry investigating the development of analytical separation methods. He also teaches ion chromatography and capillary electrophoresis at the University of Marseille.
Maëlle Bouscarel obtained a Masters in analytical chemistry at the University of Marseille in 2005. She now works in a laboratory that controls horse doping.
References
1. K.E. Grant et al., J. Radioanal. and Nuclear Chem., 211(2), 383–402 (1996).
2. ANDRA (French national agency for the management of nuclear wastes) — Spécification. Document : ACO SP ASRE 99.001/2 (Mai 1999).
3. A. Ringbom, Les complexes en chimie analytique, Ed Dunod, (Paris 1967).
4. L.G. Sillén, Stability constants of metal-ion complexes, The Chemical Society, Burlington House, London, UK.
5. W. Buchberger and S. Mülleder, Mikrochimica Acta., 119(1–2), 103–111 (1995).
6. G. Owens et al., Environmental Science and Technology, 34, 885–891 (2000).
7. A. Padarauskas and G. Schwedt, J. Chromatogr. A, 773, 351–360(1997).
8. P.-L. Laamanen, Anal. Bioanal. Chem., 381(6), 1264–1271 (2005).
9. R.N. Collins et al., Environmental Science and Technology, 35(12), 2589–2593 (2001).
10. M.L. Miller et al., J. Anal. Toxicol., 21(7), 521–528 (1997).
11. A. Dodi and V. Monnier, J. Chromatogr. A, 1032, 87–92 (2004).
12. DIN 38413-8: 200-09 ICS 13.060.50. German standard methods for the examination of water, wastewater and sludge individual constituents (group P). Part 8: Determination of nitriloacetic acid (NTA), ethylenediamine tetraacetic acid (EDTA) and diethylenetriamine pentaacetic acid (DTPA) by liquid chromatography (P8).
13. Macherey Nagel, Application note N° 119780: Complexing agents.
14. AFNOR, NF EN 13368–1 Determination des agents chélatants dans les engrais par chromatographie ionique Partie 1: EDTA, HEDTA et DTPA Ed AFNOR (2001).
15. Dionex, Document N° 031299-05, 7 November 2002.
16. AFNOR, NF ISO 16588 Qualité de l'eau — Dosage de six agents complexants — Méthode par chromatographie gazeuse, Ed AFNOR (2004).
17. M. Sillanpää, J. Sorvari and M.L. Sihvonen, Chromatographia, 42, 578–582 (1996).
18. B. Nowack and J.M. Von Briesen, Biogeochemistry of chelating agents. American Chemical Society, Washington, DC: 1–18.
19. Standard AFNOR XP T 90-210. Protocole d'évaluation d'une méthode alternative d'analyse physico-chimique quantitative par rapport à une méthode de référence. Ed AFNOR (1999).
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.











