Supercritical Fluid Chromatography (SFC): A Review of Technical Developments
In the mid 1980s the chromatographic application of gases compressed (or liquefied) to supercritical state was tested by research teams from several analytical instrument manufacturers. The recently introduced capillary columns seemed ideal candidates on which to perform such supercritical fluid chromatography (SFC) methods.
Capillary SFC
In the mid 1980s the chromatographic application of gases compressed (or liquefied) to supercritical state was tested by research teams from several analytical instrument manufacturers. The recently introduced capillary columns seemed ideal candidates on which to perform such supercritical fluid chromatography (SFC) methods. In addition, gas chromatography (GC) detectors, including flame ionization, electron capture and nitrogen phosphorous, were optimized for SFC with additional heating zones to take into account adiabatic cooling during the decompression phase. In these early days, the targeted application areas were primarily in the petrochemical industry. It is interesting to note that most pioneers from the pharmaceutical industry who tested the available instruments found the technology very limited, if not almost useless. As a consequence of this, in the early 1990s, the technology almost died through lack of application to the pharmaceutical market.

Denis Berger
Packed-Column SFC
In 1995 Terry Berger acquired the Hewlett-Packard SFC product line and, with a few colleagues, founded Berger Instruments Inc. The company began by modifying the only available analytical SFC instrument to produce the first modular packed-column SFC system dedicated to pharmaceutical applications. This instrument demonstrated resolution similar to, if not better than, reversed-phase HPLC, and represented a rebirth of normal-phase chromatography. By taking advantage of the very low viscosity and high diffusivity of the mobile phase — CO2 and modifier (usually methanol) — very fast column equilibration and higher optimum flow-rates were possible, and very long columns (up to 8 × 25 cm) packed with smaller particles, often in super-optimum flow-rates, could be used. The ability to use optimized GC detectors was maintained and some SFC users even developed interfaces (or specific cells) to collect simultaneous data from multiple detectors in single runs, including diode array, mass spectrometry, evaporative light scattering, atomic emission, and Fourier transform infra red spectroscopy. The recently introduced Corona CAD (ESA Inc., Chelmsford, Massachusetts, USA) has also been successfully tested.
Terry Berger was awarded the 2004 Martin Gold Medal by The Chromatographic Society, and the following is an excerpt from their website:1
"Dr Berger is considered by many to be the father of modern supercritical fluid chromatography (SFC). Starting in 1985, he spent more than a decade systematically undoing many of the misconceptions about packed-column SFC. In the process he deconvoluted density and solvent strength effects. He showed that, contrary to several proposed theories, very long columns with large pressure drops were feasible. He introduced the use of additives and systematically studied their effects on peak shape and retention. He demonstrated the separation of broad classes of compounds and, against the common perception, showed that packed-column SFC was broadly applicable to small drug-like molecules. He specified the feature set of the Hewlett Packard SFC G1205A, which was commercially released in 1992. More recently he led a team in the development of separator technology, which allows quantitative recovery of solutes without cyclone separators or aerosol generation. This development won a R&D 100 award as one of the 100 most important technical developments of 2000."

Analytical SFC diagram
This later technological breakthrough sparked the interest of R&D chromatographers from large pharmaceutical corporations, and encouraged development of the first preparative SFC system optimized for purification of compound libraries from combinatorial chemistry programmes.
Preparative SFC
The main problem encountered in the development of a preparative SFC prototype, as required for high-throughput purification of CombiChem libraries, was the technology available to collect fractions. The cyclone technology did not allow for the building of a self-cleaning system able to process up to 12 samples per hour (5 min runs, including system cleaning and column recycling between injections). The phase separation technology was then developed, which controls the gentle decompression of CO2 to a gaseous phase just beyond the electronic backpressure regulator without aerosol formation, thus pushing a film of modifier along the capillary walls to the fraction selection valve. The eluted compounds remain soluble in the modifier liquid phase, and peak collection is driven by an electronic peak detector analysing the signal from a fast-response detector (e.g., UV/vis).
One of the key successes of preparative SFC has been its impact (higher speed and resolution, higher productivity at lower operational costs) on the chiral separations market. Achievements have exceeded expectations in this field and led to another significant development: the production of larger amounts (from milligrams to kilograms) of pure enantiomers. This has been optimized by both stacked-mode injection, which decreased the overall time to purify large amounts of pure enantiomers from a clean racemic mixture (by multiple injections of the same sample before the end of a run), and by preparative mode injection system (installing the sample loop within the modifier stream before the modifier/CO2 mixing point, thus allowing large-volume injections without impact of sample solvent onto column equilibrium). Technology for peak collection at nearly atmospheric pressure (< 0.07 bar) has also decreased the complexity of instrument plumbing and improved operational safety.
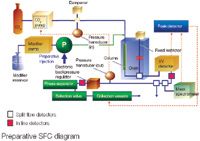
Preparative SFC diagram
Optimization of these technologies has led to the development of successful preparative instruments, which perform at their best using isocratic elution for chiral separations, but still remain effective when operated with gradient elution in sequential runs. The up scaling from analytical to preparative SFC instruments has also improved (overlapped chromatograms when respective flow-rates are kept proportional to column sections) thanks to features such as the dynamic compensation of variable compressibility at all achievable gradient compositions, pressures, temperatures and flow-rates.
At the analytical scale, an improved high-pressure cell for the diode array detector has been developed to increase signal (light path increased from 6 to 10 mm) and decrease noise (thermal equilibration of the mobile phase reaching the optical cell). Such a 5–10 times improvement in the signal-to-noise ratio, depending on SFC instrument operation parameters, has been welcome by chromatographers who perform quantitative analysis, especially in a cGLP/cGMP environments.
What Does the Future Hold?
The most demanded feature for preparative SFC has been a kind of "mass-directed fraction collection", which could perform reliably for all compounds of interest. This has not been technically feasible because none of the available ionization methods (either APCI or ESI) could guarantee such a successful detection. Therefore, until recently, the proposed alternative has been the initial analysis of samples using SFC–MS, followed by the transfer of sample table and analysis results to the preparative instrument. Subsequently, purification is performed based on the retention time of the peak identified by its targeted mass, and recovery is based on the UV/vis signal from the preparative instrument.
Agilent recently released the 6100 series of mass detectors, which can rapidly scan the targeted mass range using both APCI and ESI interfaces concomitantly, and switching continuously between positive and negative ion modes. Therefore, the risk of missing a targeted compound is certainly minimized, offering the opportunity to develop the first mass-directed preparative SFC instrument.
Conclusions
In all analytical and preparative applications, SFC can achieve results comparable (speed, resolution, throughput, productivity) to those offered by UPLC at analytical scale only; and this at pressure never exceeding 400 bar, even if sub-2 µm particles are used to pack the columns. The lower viscosity of the mobile phases used in SFC is directly responsible for this, and also allows the use of over-optimum flow-rates in preparative work without exceeding a reasonable column pressure drop. Finally, the downstream processing of collected fractions from preparative SFC (solvent evaporation, waste management) is more economical in terms of energy, time and money. These recognized benefits will certainly ensure a bright future for SFC, especially in the fast growing field of chiral separations.
Reference
1. http://www.chromsoc.com/biographies.html
After 15 years of research in analytical and preparative (bio)chromatography, Denis Berger joined Hewlett-Packard as the European bioscience business manager. In 1995, he founded his own company, Chemputeam SA, and distributed the SFC systems from Berger Instruments Inc. in Europe. He has now been appointed by Mettler-Toledo AutoChem as a senior technology and applications consultant to further develop the Berger SFC European market.
Denis Berger, Berger SFC, Mettler-Toledo AutoChem, Newark, Delaware, USA.

Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.











