Setting Realistic Expectations for GC Optimization
LCGC Europe
Setting realistic expectations requires a good working knowledge of an instrument's capabilities in terms of the sample requirements as well as an understanding of the effects of real-world samples and their matrices on long-term instrument performance.
Chromatographers have high expectations of their instrumentation. They want to see an instrument system deliver high performance levels for their individual samples, and if it does not, they want to know why. Manufacturers write instrument specifications for specific test and evaluation samples under specific analytical conditions. Real-world samples and instrument conditions seldom closely resemble the test set-up, so the manufacturers' examples often set analysts' expectation levels too high. Instrument specifications are better thought of as a basis for comparing two or more instrument systems before purchase, much as car buyers might compare the mileage rating or horsepower of several competitive models.
Even if chromatographers were to verify overall instrument system performance by duplicating the manufacturer's test protocols, the instrument might not deliver that level of performance for their specific samples. They should consider any chromatographic sample in terms of its relationship to the instrument subcomponents — inlet, column, detector and data handling — when setting performance expectations. A careful analysis of a sample as it passes through each instrument subcomponent before committing to a particular set of instrument options and accessories will help tremendously to reduce or eliminate surprises and disappointments later.
Even when a system is already in use, and its performance is less than desired, this type of analysis will point out problem areas that chromatographers can adjust and optimize for the specific analytical problem at hand.
Optimization Goals
Successful optimization requires realistic expectations and a clear understanding of the goals to be met. Optimization, whether of the hardware or the gas chromatography (GC) method, takes two forms. The first and most common form involves adjustment of operating parameters to achieve improved performance. The second form buys better performance through equipment or column upgrades. Chromatographers should expect their instrumentation to deliver the performance of which it is capable, but they should also realize that going beyond that level might require investment in upgraded or new equipment. Strong interdependence of the various instrument components on each other often makes major upgrades expensive or impractical, especially in GC. For example, converting a packed-column instrument to capillary-column capabilities or going from conventional to high-speed capillary column performance may require the replacement of most of the instrument components, from the pneumatics through to the detector. In such situations, a new instrument might be the best solution. Conversely, adding a selective detector or an on-column inlet to an existing system capable of accepting such new options is a reasonable upgrade that adds capabilities without requiring extensive modifications.
The best opportunities for hardware optimization exist when instrumentation fails to deliver desired performance goals that lie within its capabilities. For example, an improperly adjusted or configured split-splitless inlet may compromise results' accuracy and repeatability. Adjusting an inlet's operating conditions and configuration might establish acceptable performance but only if the inlet can deliver that performance in the context of the specific sample, its components and their concentrations. In addition, the column must accept the sample without compromising resolution or quantification as it comes from the inlet. Analytes must be eluted from the column with mass levels and peak profiles that do not exceed the detector's capabilities, and the data-handling system must process the raw chromatographic data accurately and consistently from run to run. Unrealistic expectations cause a perceived problem that requires either an adjustment of the expectations or the installation of hardware upgrades. The majority of operational problems arise when the analytical components fail to deliver the performance of which they are capable; improper set-up or operation causes most of these difficulties.
Set Realistic Expectations
Often, problems arise because analysts fail to set performance expectations within the capabilities of the instrument. Ideally, analytical requirements will establish the necessary level of instrument performance, which will determine the feasibility for a specific instrument package to meet the analytical challenge. With an unlimited budget, an analytical laboratory could obtain various instruments, each suited to a particular analysis or set of analyses. In reality, however, instruments are often tasked with samples that demand too much. For instance, an instrument system might not have the necessary sensitivity, or chemically active components might cause side effects in the column. Peaks may be eluted too close together or the pneumatic requirements for a column may exceed an instrument's capabilities. Concentrations of all components of interest and the injected volume should fall within the operating range of the instrument system. Inlet systems and columns only tolerate a limited injection volume without invoking specialized large-volume injection techniques. Polar and chemically active components may encounter a restricted operating range because of secondary effects from adsorption or breakdown in the inlet or column. Column overloading affects some components at lower amounts and temperatures than it does at others.
Analysts sometimes try to force an instrument system to deliver a separation that is outside its normal operating range, which usually results in trouble. Knowing and understanding instrumentation design and performance limits — and staying within those limits — helps analysts avoid problems and understand difficulties when they do occur. Setting realistic expectations requires a good working knowledge of an instrument's capabilities in terms of the sample requirements as well as an understanding of the effects of real-world samples and their matrices on long-term instrument performance. Often chromatographers fail to examine the entire sample path through the instrument from inlet to detector.

Table 1: Suggested inlet parameter operating limits.
Evaluate the Operating Range
GC users should critically examine the relationship of their samples to the operating range of their instrument system. If the sample lies outside this range, a different injection technique, column, or detector may be appropriate. To determine how well matched the sample and instrument are, first ascertain the highest and lowest analyte concentrations in the sample, as well as the nature of any chemically or thermally active components. Next, assess the upper and lower limits within which the column will pass a sample easily without loss of trace-level components or loss of peak resolution caused by overloading. Then obtain the manufacturer's detector specifications, which should list detector sensitivity or minimum detectable amount, detector dynamic range, and some information about compatibility with packed or capillary columns. With this information on hand, evaluate whether the sample component amounts will exceed or undershoot the normal operating ranges of the inlet, column and detector.
Inlets: Figure 1 illustrates the interrelationship between solute amounts and inlet, column, and detector considerations.1-3 The dashed lines illustrate its use for 1 µL split injections at minimum and maximum sample concentrations of 1 ng/µL (1 ppm) and 100 µg/µL (100 parts per thousand or 10%), respectively. This range is a very wide sample dynamic range that analysts would rarely encounter, but it will serve the purposes of this example. Here, a split ratio of 100:1 reduces the minimum and maximum amounts entering the column by a factor of 100 to 10 pg and 1 µg, respectively. A different split ratio will change the slope of the slanted lines. To construct similar lines for other split-injection situations, choose the minimum and maximum analyte amounts or concentrations per microlitre of sample at the top of the figure on the split scale. Draw two slanted lines that connect the minimum and maximum injected analyte amounts on the split scale with the corresponding amounts divided by the split ratio on the lower splitless scale. Next, draw vertical lines connecting the lower ends of the slanted lines on the lower splitless scale with the same amounts on the bottom grams scale. In the figure, these lines are drawn at 10–11 and 10–6 g, which correspond to the minimum and maximum solute levels in the example sample after splitting 100:1.
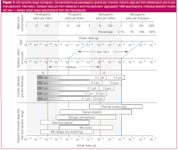
Figure 1
For splitless injection, Figure 1 shows corresponding minimum and maximum solute amounts of 100 fg/µL (100 parts per trillion) and 10 ng/µL (10 ppm). To construct similar lines for other samples, draw vertical lines connecting the minimum and maximum solute amounts on the splitless scale on top with the same amounts on the bottom grams scale and ignore the split scale. In the figure, these lines are drawn at 10–13 and 10–8 g, which correspond to the minimum and maximum solute levels in the example sample without splitting. For either split or splitless injection, the left- and right-hand lines delineate the minimum and maximum solute amounts entering the column from the inlet system. If these limits do not lie within acceptable limits for the column and detector, problems may arise with column overloading, solute adsorption or decomposition, detector sensitivity or detector overloading.
Analysts can adjust inlet parameters or the injection technique itself to increase or decrease solute amounts entering the column. If analyte levels entering the column are too high, increasing the split ratio will adjust the amounts downward until they fall within the range of operation of the rest of the instrument system. For too low levels, increasing the sample volume in splitless injection will boost the amounts entering the column. Chromatographers must observe certain operating limits to the split ratio and injected volume or they risk losing accuracy, precision and peak resolution. Table 1 lists some of these parameters with suggested upper and lower practical limits. Some specialized injection techniques such as large-volume injection enable injection sizes of 100 µL or greater; a previous "GC Connections" addressed this technique.4
Columns: Now, move down to the column diameter and film thickness portion of the figure and find the column configuration that is closest to the column in use. The grey areas on the left-hand side of each bar represent injected amounts less than 10 pg. At these levels and less, solute adsorption and breakdown can become a significant problem, not only in the column but also in the inlet. Certain solutes — free acids and bases, alcohols and other polar substances — will exhibit some degree of adsorption at much higher levels. The extent of these effects depends strongly on the chemical nature of the solute in question, column quality, stationary-phase film thickness (df) and chemistry, injection technique, and inlet cleanliness and deactivation. It is sometimes difficult to distinguish inlet and column effects without performing an additional experiment on a separate inlet or column to determine the problem source.
In the other direction, the right-hand sides of the column bars represent typical solute amounts — the sample capacity — above which significant peak distortion may detract from available peak resolution. The lower limit for the stationary-phase film thickness of 0.1 µm and the upper limits of 0.5, 1.0 or 5.0 µm represent a sampling of commercially available columns. Speciality columns may have other configurations. Column length also plays a role in the sample capacity: longer columns tend to accept higher solute amounts than shorter ones do. However, this effect is not large. Column temperature is also important; columns will tolerate greater solute amounts at higher temperatures.
Thicker stationary-phase films and larger column inner diameters support higher solute amounts without distorting peak shapes. At the same time, however, those columns deliver fewer theoretical plates because of increased diffusion and resistance to mass transfer in the larger mobile- and stationary-phase volumes. Gas chromatographers usually choose first to adjust sample amounts by changing the injection conditions. If that approach fails to eliminate column overloading, they might have to use a thicker film column. A larger inner diameter might solve the problem, but this measure should only be used as a last resort.
Detectors: To evaluate detector suitability, follow the minimum and maximum solute amount lines down to the detector section of Figure 1. The minimum solute amount should be significantly higher than the minimum detection limit indicated in the figure for the detector in use. The minimum detection limits in the figure reflect solute amounts that should produce at least a 10:1 signal-to-noise ratio (S/N) for the most sensitive detector models, as listed in recent manufacturers' specifications. Not all detectors will deliver this sensitivity, so be sure to check the specifications for the exact detector in use. Preferably, analysts should measure individual detector sensitivity and S/N with a known amount of test substance so that they can accurately gauge this lower limit. The detector must be in good working order as well. (See the June 2000 instalment of "GC Connections" for a discussion of S/N1 ).
On the high end, most detectors — with the exception of the electron-capture detector and the mass spectrometry (MS) detector in the single-ion monitoring mode — produce linear responses at levels well above the column overloading point for capillary columns. Thus, the column will usually impose an upper limit that lies within the detector's normal operating range. When operating electron-capture detectors and MS detectors in single-ion monitoring mode, however, chromatographers need to be aware that even though peak shapes can be normal at higher solute levels, the detector may not respond linearly to increasing amounts. Here, as well, a careful evaluation of a detector's response across its intended dynamic range for the specific sample in question will reveal potential problems.
If the minimum and maximum solute amounts lie outside a detector's normal operating range, consider adjusting the amount of sample that enters the column by modifying injection parameters such as split ratio or injected amount. Sometimes it makes more sense to change the injection technique or even to consider a different sample preparation procedure to bring the minimum solute amounts up to useful levels.
Finally, not all detector electronics can respond to the entire detector dynamic output range at one setting. Usually this response is not an issue unless the sample itself spans a wide dynamic range of solute concentration or the detector range and attenuation settings are configured incorrectly. Flat-topped peaks present strong evidence of this situation, which must be corrected to achieve good quantification.
A flame ionization detector, for example, responds linearly over at least six orders of magnitude. Purely analogue detector electronics, however, may not cover such a wide range without a means to change range during an analysis. Digital electronics require a working range of at least 20 binary bits to reproduce six orders of magnitude: 220 = 1.04 × 106 . A 24-bit range is preferable: 224 = 1.67 × 107 . Even though they can output a very wide signal range, digital systems rely on analogue components close to the detector itself, and so they are limited by the analogue electronics' capabilities. Schemes such as autoranging electronics or logarithmic amplifiers help to increase the analogue section's performance. Chromatographers need to be aware of these issues to the extent that they can identify a possible problem situation and take corrective action as required.
Look at the Whole Picture
Figure 1 presents a way for chromatographers to look at the whole instrument system in the context of their samples. By following the minimum and maximum solute amounts through the inlet system, column and detector, GC users can quickly evaluate the suitability of their instrument system for a particular analysis.
The high end: The example solute amount lines in the figure delineate the upper and lower limits of GC analysis on capillary columns with standard split–splitless, on-column or direct inlets. At the high end, a 10% sample will require split injection at a 100:1 ratio or more to bring solute amounts to less than 1 µg for wide-bore column use. A wide-bore, 530 µm i.d. column consumes approximately 3–5 mL/min of carrier gas at normal linear gas velocities; a 100:1 split ratio on this type of column will need a 300–500 mL/min split vent flow. This operation is possible if the column is long enough to create a high enough pressure drop so that the inlet system can deliver the vent flow. The required pressure drops of at least 20–35 kPa (3–5 psig) require column lengths of 30 m or more for a 530 µm i.d. column. Shorter wide-bore columns can cause problems if the split inlet pressure is less than 20 kPa, because the natural pressure drops through the inlet system itself under high split flow-rates may force the pressure at the column to be greater. Depending on the inlet pneumatic design, the column will operate at flow-rates higher than apparent from the set point pressure, or the inlet system will fail to reach the pressure set point and will hold the instrument system in an unready state.
If the analysis requires more resolution than a wide-bore column can deliver, narrower 320 or 250 µm i.d. columns will require even higher split ratios to bring solute amounts down to their level. A 320 µm i.d. column remains feasible, but the capability of an inlet splitter to accommodate highly concentrated samples for injection on narrower-bore columns is limited. Typical flow-rates for a 250 µm i.d. column are in the 1–2 mL/min range. At the maximum split vent flow of 300–500 mL/min, this column translates to split ratios of 150:1 to 500:1. These split ratios are barely sufficient to bring the solute amount entering the column to the 500 ng level that a 250 µm i.d. column with a relatively thick 1.0 µm stationary-phase film will tolerate. The more commonly used thin-film 250 µm i.d. columns with 0.25–0.50 µm films require another approach to bring solute amounts to less than 100 ng. Very small liquid sample injection volumes suffice, but relative standard deviations for injected amounts less than 0.5 µL tend to be worse than for higher injected amounts. Most chromatographers choose to dilute the sample with a noninterfering solvent as a way to bring solute concentrations to levels that require less extreme split inlet vent flow-rates.
Splitless, on-column, or direct injection of samples that contain 0.1–1 µg solute amounts or less is practical on thick-film 320 µm or 530 µm i.d. columns, as shown in Figure 1. Higher solute concentrations will lead to column overloading. Narrower bore columns, however, will not tolerate those high solute amounts, so that sample dilution again may be appropriate in this situation. Ideally, split injection is the right choice at such high concentrations, but sometimes the sample requirements dictate otherwise.
On the high end, only the flame ionization and thermal-conductivity detectors possess sufficient linearity at the top of their ranges to make them useful for these concentrated samples. The selective and MS detectors will be severely overloaded by solute amounts greater than 100 ng or greater than 10 ng in the case of the electron-capture detector. Thus, even if the column tolerates high solute levels, the detector requirements might determine the best injection technique or they might dictate adding a sample dilution step before injection to bring injected quantities within the optimal operating range.
The middle range: The overlap between split and nonsplit injection techniques covers approximately five orders of magnitude. Chromatographers have a lot more latitude for matching injection techniques, column configurations and detector ranges in this middle range — from 10 pg to 100 ng. The injection technique of choice will be split injection for solute concentrations greater than 10 ng/µL and splitless, direct or on-column injection for concentrations less than that level. A direct or on-column inlet will work at the upper end of this range, as well as for situations in which a split inlet is not available. Nearly all columns should accommodate these solute levels without much trouble. At the 10-100 pg level, caution is merited when using split injection for polar solutes or for those that tend to decompose under the influence of exposed active surfaces. Most selective detectors, such as the nitrogen-phosphorus, photoionization and flame photometric detectors as well as the flame ionization and MS detectors, function well at these levels. Electron-capture detectors and MS detectors in the single-ion monitoring mode may experience detector overload at the upper end of this range.
The low end: At the bottom end of the mass range — less than 10 pg/µL — split injection can reduce solute amounts to levels that will experience problems with passage through some columns or with detection on many detectors. Splitless, direct or on-column injection ensures that as much solute as possible gets onto the column to minimize problems arising from adsorption and decomposition in the inlet or the column. Remember, however, that too-large injection volumes can cause solvent flooding or other problems in the inlet or column that lead to peak distortion and resolution loss. Solutes in this low concentration range benefit the most from additional sample preparation steps that increase their concentrations or from specialized large-volume injection techniques.
Column bleed can become a problem with low-concentration solutes. Column bleed appears as an increasing baseline during temperature-programmed elution; higher noise levels also result from column bleed. The baseline offset and noise from column bleed will reduce the signal-to-noise ratio, which will detract from a detector's available minimum detection limit. Column bleed can also cause the data-handling system to have trouble with correctly assigning peak start, apex and end points, which can cause reduced precision and accuracy. Most column manufacturers offer a line of high-performance, low-bleed columns that chromatographers should choose in this situation.
Detectors with high sensitivity, such as electron-capture detectors or MS detectors in the single-ion monitoring mode, should have no difficulty operating in the low range. Others, such as flame ionization and nitrogen–phosphorus detectors, will be hard pressed to perform well at levels less than 1 pg. Analysts must take special care to ensure system cleanliness and freedom from gas contamination at these low solute levels, no matter which detector they use. Ferrules, O-rings and other seals can contribute significantly to detector background at these levels. Systems previously used at high solute levels may require a thorough cleaning before an instrument will deliver its specified performance on the low end. Carrier gas and detector make-up or flame gases must be ultrahigh-purity grade. Appropriate pressure regulators, clean tubing and gas filtration as close as possible to the instrument are essential, even though the gas supply is of high quality, because minute leaks at gas line connections or traces of organic material in the tubing or regulators will destroy the best quality gas.
Conclusion
GC instruments accommodate an extremely wide range of solute concentrations. Although any one analysis is unlikely to demand that the instrument deliver high performance across its entire operating range at once, the minimum and maximum solute amounts in a sample must lie within the available dynamic ranges for the inlet, column and detector. Straying outside the range of any one subcomponent invites trouble with peak resolution or analytical accuracy and precision. The correct choice of sample preparation and injection techniques, matched with an appropriate column and detector, helps ensure the reliability and longevity of the instrument components and the quality of the analytical results.
References
1. J.V. Hinshaw, LCGC Eur., 13(6), 410–417 (2000).
2. K.J. Hyver, Ed., in High-Resolution Gas Chromatography (Hewlett-Packard Co., Avondale, Pennsylvania, USA, 3rd ed., 1989), pp. 1–21.
3. W. Seferovic, J.V. Hinshaw Jr. and L.S. Ettre, J. Chromatogr. Sci., 24, 374–382 (1986).
4. J.V. Hinshaw, LCGC N. Am., 12(7), 526–530 (1994).
5. H.M. McNair and James M. Miller, Basic Gas Chromatography, (John Wiley & Sons, Inc., New York, 1998), p. 123.
"GC Connections" editor John V. Hinshaw is senior staff engineer at Serveron Corp., Hillsboro, Oregon, USA and a member of the Editorial Advisory Board for LCGC Europe.
Direct correspondence about this column to "GC Connections", LCGC Europe, Advanstar House, Park West, Sealand Road, Chester CH1 4RN, UK, e-mail: dhills@advanstar.com
For an ongoing discussion of GC issues with John Hinshaw and other chromatographers, visit the Chromatography Forum discussion group at http://www.chromforum.com

Study Examines Impact of Zwitterionic Liquid Structures on Volatile Carboxylic Acid Separation in GC
March 28th 2025Iowa State University researchers evaluated imidazolium-based ZILs with sulfonate and triflimide anions to understand the influence of ZILs’ chemical structures on polar analyte separation.
Quantifying Microplastics in Meconium Samples Using Pyrolysis–GC-MS
March 26th 2025Using pyrolysis-gas chromatography and mass spectrometry, scientists from Fudan University and the Putuo District Center for Disease Control and Prevention detected and quantified microplastics in newborn stool samples.













