Sulphur and Halide Determination by Combustion Ion Chromatography
The fully automated combustion ion chromatography (CIC) system presented here combines a highly efficient combustion system with the separation power of ion chromatography (IC).
C. Emmenegger, A. Wille, and A. Steinbach, Metrohm International Headquarters
The fully automated combustion ion chromatography (CIC) system presented here combines a highly efficient combustion system with the separation power of ion chromatography (IC). CIC allows for the simultaneous, speciated trace analysis of halide (F, Cl, Br, and I) and sulfur compounds (as sulfate) from sub-ppm to percent levels in any nonaqueous sample matrix.
Chloride and sulfur recoveries in the certified polymer standard ERM-EC681k and a S-benzyl thiouronium chloride sample were between 96–103% and 97–101%, respectively. While sulfur concentrations in the diesel fuel and the unleaded gasoline 95 sample were around 10 mg/kg, the biodiesel sample showed a lower sulfur concentration of 3.8 mg/kg. Chloride concentrations in all fuel samples ranged between 4 and 8 mg/kg. Because multiple burns can be collected in the same absorption solution — demonstrated by means of chloride and sulfate data (R2 ≥ 0.998) — CIC achieves detection limits in the sub-ppm range.
In response to the decreasing lifetime and the skyrocketing production of electronic and electrical equipment, the "restriction of the use of certain hazardous substances in electrical and electronic equipment" (RoHS) limits the content of some hazardous species, such as polybrominated biphenyls, in these types of equipment. The electronics industry, driven by several international regulations, is striving to obtain halogen-free products. Because of upcoming international regulations, the rapid monitoring of partly corrosive and toxic organohalogen compounds in challenging matrices such as coal, crude oil, naphtha, liquefied petroleum gas, and so forth, will gain importance.
The most prominent techniques for halogen determination in these materials include the determination of absorbable/total organic halogens (AOX/TOX), inductively coupled plasma mass spectrometry (ICP–MS), ion-selective electrodes (ISE), and ion chromatography (IC). However, either results are unspeciated sum parameters, or samples are difficult to bring into solution and thus require complex and time-consuming sample preparation procedures.
These drawbacks can be overcome by using combustion IC (CIC). This presentation highlights the applicability of automated CIC to the determination of trace halide and sulfur compounds in different sample matrices.
Experimental
Instrumentation
The fully automated combustion ion chromatography system consists of the following devices:
- 881 Compact IC pro – Anion – MCS
- Mitsubishi ABC-100
- Mitsubishi AQF-100
- Mitsubishi GA-100
While the ABC-100 transfers the samples into the furnace (AQF 100), the GA-100 absorbs the gaseous compounds produced and transfers the resulting solution to the ion chromatograph. The combustion unit is controlled by the system software of Mitsubishi's AQF-100. Operational parameters for the combustion and the subsequent chromatographic separation are indicated in Table I.

Table I: Operational parameters for the determination of chloride, bromide and sulfur by CIC
Standard solution, gases, and eluents
All reagents used in this work were of the highest purity grade (puriss. p.a.). Oxygen and argon gas were purchased from Pangas (Dagmersellen, Switzerland) and have a purity better than 99.999 and 99.996% (v/v), respectively. All solutions were prepared with deionized water with a specific resistance higher than 18 MΩ·cm. The polymer certified reference material ERM-EC681k was purchased from the Institute for European Reference Materials (ERM) in Geel, Belgium. S-benzyl thiouronium chloride stems from Merck Schuchardt OHG in Hohenbrunn, Germany. The fuel samples were purchased from several Swiss gas stations.
Principle
According to Figure 1, sample pyrolysis takes place in the combustion system. Combustible samples are oxidized by O2 at temperatures above 900 °C. While sulfur-containing compounds are oxidized to sulfur dioxide, halogen-containing compounds are converted to hydrogen halides or gaseous halogens. The gaseous compounds formed are led into an oxidizing absorption solution, where sulfur dioxide is further oxidized to form sulfate and the halogens form halide ions. The absorption solution is then fed into the sample loop of the Metrohm 881 Compact IC pro. All kinds of combustible samples can be analysed.
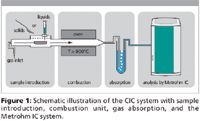
Figure 1
While one chromatogram is being recorded, pyrolysis of the next sample is already under way. The MagIC Net software reliably controls the analysis and automatically calculates the sulfur and mass fractions for each sample. Both liquid and solid samples can be analysed efficiently in a short time by using the sample changer.
Halide and sulfur determination
(a) in a certified polymer standard
The certified polymer reference material is a low-density polyethylene granulate spiked with known amounts of chloride, bromide, and sulfur (Table II). It is used to test the performance of the CIC unit.

Table II: Certified and experimentally determined chloride, bromide and sulfur concentrations of the certified polymer standard ERM-EC681k
The results for chloride, bromide, and sulfur (Figure 2) are well within the certified concentrations of the ERM-EC681k standard. Recoveries between 96 and 103% demonstrate complete matrix destruction during combustion and the quantitative capture of gaseous chloride-, bromide-, and sulfur-containing compounds by the absorption solution.
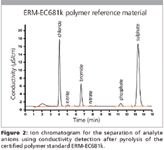
Figure 2
(b) in S-benzyl thiouronium chloride
A known amount of S-benzyl thiouronium chloride was automatically combusted and analysed for its chloride and sulfur content (Figure 3). Recoveries between 97 and 101% demonstrate the applicability of the combustion procedure (Table III).
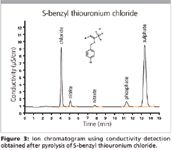
Figure 3
(c) in different fuel samples
Sulfur in fossil fuels is present in both inorganic and organic form. While the first fraction mainly affects the motor performance in terms of precipitating salts, organosulfur compounds, when combusted, emit detrimental sulfur dioxide with severe adverse environmental impacts. In view of these drawbacks, the sulfur content, whether in the inorganic or in the chemically bound organic form, has to be controlled. Whereas the water-soluble inorganic ions can be selectively determined by IC coupled to upstream in-line dialysis or extraction, the total sulfur content can be determined by CIC.

Table III: Theoretical and experimentally determined chloride and sulfur contents of S-benzyl thiouronium chloride
Chloride concentration in all investigated fuels range between 4 and 8 mg/L (Figure 4, Table IV). While sulfur concentrations in the unleaded gasoline and the two diesel samples are approximately 10 mg/kg, the sulfur content of the biodiesel sample is significantly lower (3.8 mg/kg). When multiple combustions are collected in the same absorption solution, as displayed in Figure 5, there is a linear correlation between the concentration of the combustion products in the absorption solution and the number of performed combustion cycles. By collecting multiple "burns" into a single absorption solution, combustion IC achieves detection limits in the sub-ppm range.

Figure 4
Conclusions
The automated CIC system allows straightforward in-line combustion and is designed to quantitatively trap the gaseous combustion products in an oxidizing absorption solution for subsequent ion chromatographic analysis. The method excels by the possibility to simultaneously determine sulfur and each individual halogen (fluorine, chlorine, bromine, and iodine) with high precision and accuracy. Sample enrichment is achieved through the possibility to trap analytes from multiple combustion cycles in the same absorption solvent. CIC is capable to determine sulfur and total speciated halides down to 0.5 ppm.
Table IV: Chloride and sulfur contents in different fuel types determined by CIC
Application fields include the petroleum, coal, plastic, semi-conductor, and power generation industries, which are obliged to monitor banned toxic compounds as they are sources of corrosion or poison.

Figure 5
References
(1) ASTM D 7359-08, Standard test method for total fluorine, chlorine and sulphur in aromatic hydrocarbons and their mixtures by oxidative pyrohydrolytic combustion followed by ion chromatography detection (combustion ion chromatography-CIC).
(2) E.M.M. Flores et al., Analytical Chemistry, 80, 1865–1870 (2008).
(3) G. Bogenschütz and T. Kolb, Ion chromatographic determinations of anions, cations and organic acids in biofuels, downloadable under http://products.metrohm.com (search for 8.000.6011EN).

Metrohm International Headquarters
Oberdorfstrasse 68, CH-9109 Herisau, Switzerland
tel. +41 71 353 85 04
Email: aw@metrohm.com; Website: www.metrohm.com

Assessing Thorium-Peptide Interactions Using Hydrophilic Interaction Liquid Chromatography
February 4th 2025Paris-Saclay University scientists used hydrophilic interaction liquid chromatography (HILIC) coupled to electrospray ionization mass spectrometry (ESI-MS) and inductively coupled plasma mass spectrometry (ICP-MS) to assess thorium’s interaction with peptides.



















