Call for Application Notes
LCGC is planning to publish the next issue of The Application Notebook special supplement in September. The publication will include vendor application notes that describe techniques and applications of all forms of chromatography and capillary electrophoresis that are of immediate interest to users in industry, academia, and government. If your company is interested in participating in these special supplements, contact:
LCGC is planning to publish the next issue of The Application Notebook special supplement in September. The publication will include vendor application notes that describe techniques and applications of all forms of chromatography and capillary electrophoresis that are of immediate interest to users in industry, academia, and government. If your company is interested in participating in these special supplements, contact:
Michael J. Tessalone, Group Publisher, (732) 346-3016
Edward Fantuzzi, Associate Publisher, (732) 346-3015
Stephanie Shaffer, East Coast Sales Manager, (508) 481-5885
Application Note Preparation
It is important that each company's material fits within the allotted space. The editors cannot be responsible for substantial editing or handling of application notes that deviate from the following guidelines:
Each application note page should be no more than 500 words in length and should follow the following format.
Format
- Title: short, specific, and clear
- Abstract: brief, one- or two-sentence abstract
- Introduction
- Experimental Conditions
- Results
- Conclusions
References
- Two graphic elements: one is the company logo; the other may be a sample chromatogram, figure, or table
- The company's full mailing address, telephone number, fax number, and Internet address
All text will be published in accordance with LCGC's style to maintain uniformity throughout the book. It also will be checked for grammatical accuracy, although the content will not be edited. Text should be sent in electronic format, preferably using Microsoft Word.
Figures
Refer to photographs, line drawings, and graphs in the text using arabic numerals in consecutive order (Figure 1, etc.). Company logos, line drawings, graphs, and charts must be professionally rendered and submitted as .TIF or .EPS files with a minimum resolution of 300 dpi. Lines of chromatograms must be heavy enough to remain legible after reduction. Provide peak labels and identification. Provide figure captions as part of the text, each identified by its proper number and title. If you wish to submit a figure or chromatogram, please follow the format of the sample provided below.

Figure 1
Tables
Each table should be typed as part of the main text document. Refer to tables in the text by roman numerals in consecutive order (Table I, etc.). Every table and each column within the table must have an appropriate heading. Table number and title must be placed in a continuous heading above the data presented. If you wish to submit a table, please follow the format of the sample provided below.
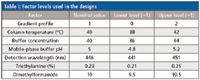
Table I: Factor levels used in the designs
References
Literature citations must be indicated by arabic numerals in parentheses. List cited references at the end in the order of their appearance. Use the following format for references:
(1) T.L. Einmann and C. Champaign, Science 387, 922–930 (1981).
The deadline for submitting application notes for the September issue of The Application Notebook is:
July 26, 2010
This opportunity is limited to advertisers in LCGC North America. For more information, contact:
Mike Tessalone at (732)346-3016, Ed Fantuzzi at (732)34603015, or Stephanie Shaffer at (508)481-5885.

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
Investigating the Protective Effects of Frankincense Oil on Wound Healing with GC–MS
April 2nd 2025Frankincense essential oil is known for its anti-inflammatory, antioxidant, and therapeutic properties. A recent study investigated the protective effects of the oil in an excision wound model in rats, focusing on oxidative stress reduction, inflammatory cytokine modulation, and caspase-3 regulation; chemical composition of the oil was analyzed using gas chromatography–mass spectrometry (GC–MS).


















