Standardized Testing of Silica as a Base Material for Difficult Bonded-Phase Preparative Applications
LCGC North America
Mechanical strength, acid and alkaline durability, loadability, and overload characteristics are a few of the parameters evaluated.
In high performance liquid chromatography (HPLC) column development, oftentimes the bonded-phase silica is only investigated to determine the suitability of a packed column for a particular separation class and not much thought is given to optimization of the base silica to make the overall packing material more suitable for the assay. Here, we examine general-purpose standardized tests to determine the quality of bulk silica before and after bonding. Base silica features such as mechanical strength, acid and alkaline durability, loadability, and overload characteristics are a few of the parameters evaluated that have an impact on the final separation and purification characteristics for a difficult test analyte such as insulin.
Despite improvements in polymeric particles, the introduction of organic–inorganic hybrids and investigations of other inorganic oxides (for example, titania and zirconia), totally porous and superficially porous silica gels still dominate as the favored base material in high performance liquid chromatography (HPLC) (1). The properties of silica, outlined in Table I, make it an ideal packing base material for bonded-phase chromatography, which is by far the most popular approach for analytical and preparative separations. For example, more than 90% of chromatographers use reversed-phase liquid chromatography (LC) in their daily work (1). Even though spherical silica has been around for several decades, manufacturers are still improving silica particles to allow them to be used for even more demanding separations and purifications.
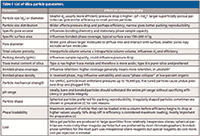
Table I: List of silica particle parameters
Although the actual number of analytical applications using bonded silica columns exceeds the number of preparative applications, for industrial purifications using porous silica-based packing materials, the amount of silica gel consumed worldwide greatly exceeds the amount of silica used to pack HPLC and ultrahigh-pressure liquid chromatography (UHPLC) analytical columns. In addition, although the properties of the packings used in preparative chromatography are similar to the optimum properties of analytical chromatography, there are some properties that are more demanding when it comes to large-scale purifications. The purpose of this installment is to discuss the properties of silica gel that must be improved further and then carefully controlled to meet the demanding applications in large-scale industrial purifications. For our test example, the purification of insulin was chosen since it is the most important large-scale process purification using bonded-phase silica gel chromatography.
The Importance of Insulin Production
Diabetes is an incurable, life-threatening disease. The increase in individuals who are and will be affected by this disease correlates with the worldwide rise in obesity and the growing number of people with access to modern medications. The number of diabetes patients is projected to balloon to a staggering 500 million in the coming years.
The most widely used medication for diabetes is insulin. This peculiar biomolecule comprises 51 amino acids with a molecular weight of a little less than 6 kDa. Insulin is considered either one of the biggest peptides or the smallest of the protein molecules. By its nature, the insulin molecule has a strong tendency to form dimers and further undergo aggregation-coupled misfolding (oligomerization) to form a cross-β assembly. This process is referred to as fibrillation and can complicate the manufacturing process as well as its chromatographic purification.
Insulin manufacturing is a standalone industry these days. Human insulin or its slightly modified variations are cloned into microorganisms. After the expression, insulin is harvested and must be rigorously purified not only from the fermentation broth residues but also from the misshapen isoform impurities. Such difficult separations can be performed using process-scale HPLC with huge dynamic-axial compression columns packed with silica-based reversed-phase stationary phases. Interestingly, the insulin manufacturing industry gobbles up a huge part of the total acetonitrile consumption of the world and almost half of the total spherical reversed-phase silica globally produced.
The biggest technological challenge for insulin manufacturers is the insulin molecules fibrillating on top of the chromatographic column, forming a tough, chewing gum–like layer that blocks the solvent flow and increases the column back pressure. Currently, the solution, though not preferable for silica gel–based packings, is to use a pH 13 sodium hydroxide solution to dissolve and remove this layer blocking the column. This procedure combined with the generally higher than average pressure of the process can damage the silica-based stationary phases badly. Thus, there is a need to develop special stationary phases to address these challenges.
Development of an Optimized Packing for Purification of Insulin
For small-scale (analytical) HPLC analysis of a given peptide (for example, the insulin molecule) typically needed at the discovery stage of a new drug development process, the most important parameter is the selectivity (separation factor) of the stationary phase used. However, when the very same peptide goes several years later into large-scale production, a whole set of new parameters become very important to make the stationary phase used in the chromatographic purification steps the best choice.
Standardized testing protocols for general chromatographic bonded phase packings abound. For example, the Tanaka test (2), the Engelhardt test (3–5), the Neue test (6), and the National Institute of Standards and Technology (NIST) and The United States Pharmacopeia (USP) test (7) among others (8,9) are generally suited for the evaluation of the separation characteristics of the bonded-phase silica packed into analytical columns and not specifically for the bare silica used in their production.
Few standardized tests have been developed for the base silica used for the preparation of chromatographic silica, especially when used for process-scale separation columns. Tests on the bulk silica before bonding can reveal underlying characteristics that may rule out a material before having it go through the bonding and characterization process. Part of the development process described here was the characterization of the base silica for attributes considered as the most important for large-scale purification. Several commercially available silicas and newly developed silicas were put through the testing process to understand the most important characteristics for large-scale insulin and peptide production. Research and development (R&D) chemists often attempt to optimize one or two packing parameters such as efficiency or resolution; however, we found that there are six important criteria for an optimized chromatography material for the preparative purification of insulin and other peptide products. Here, we cover each of these tests to determine how one might find the most suitable packings for this important application. Table II provides a list of the characteristics of the silicas tested in these experiments.

Table II: Bonded silicas and base silicas used in this study
Mechanical Strength
Nowadays, dynamic axial compression columns are widely used for preparative and process separations. Since this type of column configuration puts additional stress on a packed bed, special attention has to be paid to the mechanical stability of the silica used. The mechanical strength of the base silica is further challenged by the high pressure of operation of the packed columns, quick pressure buildup during startup, and when the high-pH washing step (described next) causes additional stress on the base particle.

Figure 1: X-ray fluorescence sample compressor used for mechanical stability test.
Two methods of testing the mechanical strength of the bare silica were used. These methods can be used by nearly everyone since the testing is straightforward and easily repeatable. Initially, the particle size distribution of the base silica gel is measured by a Coulter counter. Next, the silica particles are tightly packed into the ring of an X-ray fluorescence (XRF) spectrometer sample compressor (see Figure 1). The sample is pressed at 500 bar for 1 min. The ring is unpacked and the particle size distribution is measured again by the Coulter counter method (Figure 2). The value represented in the small table at the bottom of Figure 2 is the difference between the smaller particle percentage before and after the compression test. In this case, there was very little difference between the two tested silicas.

Figure 2: Test 1, mechanical strength of base silicas. Plot shows the particle size distribution for silica SP-100-8-PK.
A second method to measure the mechanical strength of the particle is the repeated packing and unpacking of the same silica into the same column configuration. Changes in bed length or back pressure with subsequent packings can indicate that the particles are becoming disturbed by the process of repeated packing. In this experiment, the slurry packing method was used. The packing pressure was 9.6 MPa using a mobile phase of isopropanol and flow rate of 150 mL/min. Table III shows the results of the back pressure changes for 1, 5, 10, 15, and 20 cycles of repeated packing and unpacking. For both silicas, back pressure (Table III) and bed length (results not shown) were unaffected by the packing process and, thus, both tested silicas have very similar mechanical strengths under these conditions. Although not shown, scanning electron micrographs of the unpacked silica particles after the initial, 10th, and 20th cycles also revealed that no fines were being created.

Table III: Test 2: change in back pressure with repeated dynamic axial compression packing and unpacking
Alkaline Durability Base Silica Test
As indicated earlier, in the production of insulin the use of sodium hydroxide solution is a requirement for cleaning in place (CIP) of the process column to get rid of fibrillation by-products. This requirement places severe constraints on the silica-base material as well as the bonded packing material.
Silica gel itself is soluble in water; its solubility increases with temperature and pH, as depicted in Figure 3 (10). At low pH values, the base silica gel itself is fairly stable to dissolution. At neutral pH and at high pH, dissolution of the underlying silica gel through attack by the hydroxide ion causes a loss of column performance. The degradation process is enhanced by higher temperatures. Often, a column void is formed as the base silica gel is solubilized resulting in abnormally high pressure buildup (because of particulates building up on the column outlet frit), peak doublets, peak tailing, and other undesirable characteristics. To suppress the dissolution of silica in typically used mobile phases, we must prevent the hydroxide ion in the mobile phase from reaching the underlying silica.
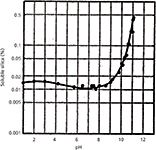
Figure 3: Solubility of silica gel in water as a function of pH.
There are several methods to increase the base stability of silica gel and bonded silica gel. One approach to accomplish this blockage is to coat the silica with a polymer that can protect the underlying silica against dissolution, provided that the mobile phase doesn't penetrate the coating and begin the dissolution process underneath the polymer shell. The polymer coating may also lead to a loss of chromatographic efficiency since diffusion in polymeric layers is generally poorer than in silica monolayer bonded phases. For high-pH work, another approach that also delays the dissolution of silica is to cover the silica gel base with a high density of chemically bonded phase. With the bonded phase coverage, the rate of silica dissolution is determined by the bonding characteristics, type of bond, type of silica, presence of certain salts and buffers, and the buffer concentration, mobile-phase pH, and other experimental parameters (11,12). Hybrid silica–organic materials offer still another approach to prevent dissolution of the packing, especially at basic pH values (13).
Another approach to increasing the alkaline durability of the base silica is to fabricate the silica to be more stable itself. Unfortunately, the procedures to make this happen are usually highly proprietary and are mostly treated by the silica companies as trade secrets. The manner of preparation, the specific surface area, the gel type, the gel time, the temperature, and the pH of the gelation step are all known procedures that affect silica solubility. Figure 4 shows the results of a study of the alkaline durability of four types of bare silica gels prepared by different methods. The tests were conducted using a 250 mm × 4.6 mm packed column with a 0.05 N sodium hydroxide solution running through it, a temperature of 25 °C, and the HPLC upper pressure limit set to 400 kg/cm2. The pressure was recorded as a function of the number of column volumes (one column volume equals 4.2 mL) that was passed through the column. For two of the silicas, less than 50 column volumes of the sodium hydroxide solution passed through the column before the pressure became catastrophic and flow through the column could no longer be achieved at the upper pressure limit. Two of the silicas showed respectable alkaline resistance and were clearly superior to the others.
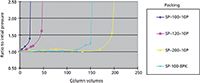
Figure 4: Alkaline durability of base silicas.
A side note: It was found in these tests that a bare silica with stronger alkaline resistance also makes a stronger bonded-phase packing material.
Alkaline Durability Bonded Silica Test
Clearly, the addition of a bonded phase should help in lessening the attack of hydroxide ion on the base silica. Depending on the type of bonding and the bonding density (or coverage), the underlying surface is more protected than the bare silica used in the previous testing. In this set of experiments, C8 siloxane phases were studied on the earlier base silicas. The same flow experiment that was conducted with the bare silica was repeated, but instead a stronger solution of 0.1 N sodium hydroxide was used to accelerate the degradation test. The conditions for the experiment were as follows:
Mobile phase: 30:70 (v/v) 0.1 N sodium hydroxide–ethanol
Flow rate: 2.0 mL/min
The remaining experimental conditions were the same as outlined for the bare silica studies above.
Figure 5 shows the experimental results obtained with C8 bonded phases. By covering the base silica with bonded phase, the stability of all silicas were improved over the bare silica lifetime (compare Figure 5 to Figure 4). In fact, the bonded silicas designated as SP-200-10-C8-BIO and SP-100-10-C8-PK showed remarkable stability in such a caustic environment. Undoubtedly, this improvement was a result of improved base silica stability coupled with improved bonding phase chemistry.
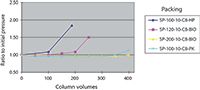
Figure 5: Plot of the relative pressure drop showing the alkaline durability of C8 bonded silicas.
Phase Loadability
Phase loadability, sometimes called sample capacity, refers to the amount of sample which can be injected onto a column without overload. It may be expressed as a milligram of sample per gram of packing basis. Overload is defined as the sample mass injected where the column efficiency falls by 10% from its normal value. Higher loadability will result in better process economy and better productivity at production scale. However, loadability is only one part of the overall preparative requirement. Loadability combined with adequate separation selectivity (covered next) and optimized retention time will lead to the highest possible productivity. Loadability is governed by the surface area of the packing material or the bonded-phase coverage.
One approach to determine loadability is to use breakthrough volume or mass. Here, a known concentration of target analyte is continuously pumped through a packed column and absorbance (or other solute parameter) is monitored at the column exit. Conditions are chosen so that the compound of interest is totally retained by the column packing. After the active sites on the column are saturated, the compound breaks through and the absorbance jumps from the stable baseline; the total volume of liquid passed through the column can then be used to calculate the total mass on that compound that was retained on the column while the liquid was being pumped through.
To measure relative phase loadability, several packings were subjected to the following conditions:
Column: 250 mm × 4.6 mm
Sample: Insulin
Sample concentration: 10 mg/mL in 10:90:0.5 (v/v/v) acetonitrile–water–trifluoroacetic acid
Detection: UV absorbance at 290 nm
Figure 6 shows the breakthrough profiles for the compound of interest (insulin) on the four different bonded-phase silicas. Each of the bonded silicas has its own breakthrough value and one (SP-100-10-C8-HP) has a somewhat higher capacity than the others with the SP-200-10-C8-BIO being of much lower capacity, which is undoubtedly related to the lower surface area of the wider pore starting silica gel.
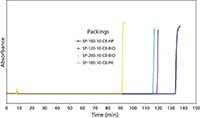
Figure 6: Determination of bonded-phase breakthrough volumes (loadability). See the text for experimental conditions.
Separation Selectivity
Separation selectivity (separation factor) is one aspect of preparative chromatography that affects compound purity. In both analytical and preparative work, selectivity is an important part of the resolution equation. It is imperative in the purification of a drug to be injected into a human body that the compound be of the highest purity possible. For insulin, a closely eluted impurity, des-amido insulin, is used to monitor the overall purity of insulin. In fact, according to the USP, the relative amount of des-amido insulin must not exceed 3% of the total amount of insulin plus des-amido insulin (14). Thus, a high separation factor between the pure insulin peak and the des-amido impurity peak indicates the quality of separation. The easiest and most reproducible way is to use standard pure insulin (reagent grade) and create an artificial impurity by simply letting the water-dissolved insulin sit on the workbench overnight, which results in the formation of the des-amido form. This binary model of insulin–insulin impurity is an ideal model for measuring separation selectivity.
To measure the separation selectivity, the following conditions were used:
SP-200-10-C8-BIO
Column dimensions: 250 mm × 4.6 mm
Mobile phase: 30:70:0.1 (v/v/v) acetonitrile–water–trifluoroacetic acid
Flow rate: 1.7 mL/min
Column temperature: 40 °C
Detection: UV absorbance at 204 nm
Sample: Insulin in 0.1% trifluoroaceitic acid in water left at 30 °C for 12 h
Sample concentration: 10 mg/mL in 0.1% trifluoroacetic acid in water
Sample volume injected: 5 µL
Figure 7 shows the chromatogram that provided a separation factor of 1.39 between the larger insulin peak and the smaller des-amido peak. This selectivity should provide a high purity of isolated insulin. Indeed, the separation factors on all of the bonded silicas were acceptable for the insulin–des-amido insulin pair.
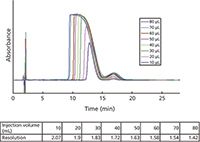
Figure 7: Test for column overload with insulin and des-amido insulin impurity. Test column: SP-200-10-C8-BIO. See the text for experimental conditions.
Column Overload
Overload is when there is a large enough sample size to cause chromatographic resolution to become unacceptable and the purity of the peak of interest is compromised. Overloading is related to the combined effect of loadability and separation selectivity. For the analytical column described above, increasingly larger injections of the des-amido plus insulin sample are made until the chromatographic resolution becomes less than 1.5. Figure 7 shows the effects of volume overloading on the chromatography using one of the test columns. As the injection volume (and mass) increases, the valley between the insulin and the des-amido insulin peak becomes higher and thus more overlap peak occurs affecting the purity of the insulin peak. For these particular injections, resolution becomes poor at the 80-µL volume (0.7 mg) and thus this is the limit of volume at the specified concentration that should be injected onto this analytical size column. For larger scale preparative and process columns, the volume (mass) injected would increase proportional to the increase in the column volume (determined by the diameter and length of the column).
Overall Comparisons of Tested Base Silicas and Bonded Silicas
In our investigations of various silica-based products for the optimized separation and purification, we looked specifically at the six most important parameters that define suitability for insulin production. These evaluation parameters may also be suitable for other peptides, but should be investigated to make sure that they are optimized for a particular peptide product. A convenient graphical method for displaying multivariant data is in the form of a two-dimensional chart of three or more quantitative variables represented on axes starting at the same point, which is called a spider diagram (also referred to as a radar chart). Figure 8 shows a spider diagram for the four bonded silica packings that were tested for insulin separation and purification. Although showing varying degrees of effectiveness, clearly one of the packings (SP-100-10-C8-PK) gave the best overall performance in insulin purification in terms of the six most important variables. Just like any chromatographic separation or method, one must compromise in achieving the best overall performance. Perhaps in a given situation, the variables may have different "weights" and those fewer variables are looked upon as the most important for that group of molecules. The most difficult parameter for insulin is the alkaline stability of base silica as well as the bonded phase. For most peptide work, the separation and purification is performed under acidic conditions using trifluoroacetic acid solutions so that alkaline stability may not be relevant. However, the acidic resistance of the bonded phase will be more important and should be included in the testing parameters.
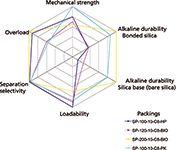
Figure 8: Spider diagrams comparing four bonded silicas for suitability for insulin purification.
Simplified standardized testing on bulk silicas as performed here is recommended as a preferred way to measure the effectiveness of packing materials for large-scale purification of various biomolecules. The tests are easy to carry out and can be performed by most laboratories using simple equipment and experimental conditions. In our opinion, so far, no test with universal application for judging bulk silica stationary phases for every species encountered has been found. The proposed standardized tests in this paper are related to the importance of the industrial scale chromatographic purification for a given molecule — insulin. The test sets will be different for a different group of molecules (for example, oligonucleotides, hydrophobic proteins, and so on) and the important variables must be determined and optimized for those molecules.
References
(1) R.E. Majors, LCGC North Am. 30(1), 20–34 (2012).
(2) K. Kimata et al., J. Chromatogr. Sci. 21, 277 (1989).
(3) H. Engelhardt, H. Low, and W. Gotzinger, J. Chromatogr. A 544, 371 (1991).
(4) H. Engelhardt, M. Arangio, and T. Lobert, LCGC Europe 10(12), 803–812 (1997).
(5) H. Engelhardt and M. Jungheim, Chromatographia 29, 59 (1990).
(6) U.D. Neue, K.V. Tran, P.C. Iraneta, and B.A. Alden, J. Sep. Sci. 26(3–4), 174–186 (2003).
(7) B. Bidlingmeyer, C.C. Chan, P. Fastino, R. Henry, P. Koerner, A.T. Maule, M.R.C. Marques, U. Neue, L. Ng, H. Pappa, L. Sander, C. Santasania, L. Snyder, and T. Wozniak, Pharmacopeial Forum 31(2), 637–645 (2005).
(8) J.J. Gilroy, J.W. Dolan, and L.R. Snyder, J. Chromatogr. A 1000, 757–778 (2003).
(9) W. Eymann, Chromatographia 45, 235 (1997).
(10) R.E. Majors, LCGC North Am. 18(12), 1214–1227 (2000).
(11) H.A. Claessens, M.A. van Straten, and J.J. Kirkland, J. Chromatogr. 728, 259 (1996).
(12) J.J. Kirkland, J. Chromatogr. Sci. 34, 309–313 (1996).
(13) U.D. Neue, J. Carmody, Y.-F. Cheng, J. O'Gara, B.A. Alden, T. Walter, E.S.P. Bouvier, and R. Crowley, "Packings with Minimal Silanol Activity," Presented at The International Symposium on High Performance Liquid Phase Separations and Related Techniques (HPLC 1999), Granada, Spain, 1999.
(14) S. Cox, Ed., Handbook of Pharmaceutical Biotechnology (Wiley-Interscience, New York, 2007), p. 485.
Keiji Koyanagi is an organic chemist, who has been working at the DAISO R&D for 12 years. He has played an instrumental role in new stationary phase development projects. His holistic view on all chromatographic parameters being interlinked was the source for the studies exploring the idea of standardized tests.

Keiji Koyanagi
Imre Sallay was born and raised in Hungary. He moved to Japan half a lifetime ago with a research grant on protein chemistry. His biochemist origin helps him to understand the needs of pharmaceutical customers using bulk silica based stationary phases. Direct correspondence about this installment to: simre@daiso.co.jp

Imre Sallay
Ronald E. Majors is the editor of "Column Watch," an analytical consultant, and a member of LCGC's editorial advisory board. Direct correspondence about this column to lcgcedit@lcgcmag.com

Ronald E. Majors

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.














