Current Practices and Considerations for a Stability-Indicating Method in Pharmaceutical Analysis
LCGC North America
What are the characteristics and relevant considerations for the development and validation of a stability-indicating method?
The demonstration of drug substance (DS) or drug product (DP) stability over the shelf life is a regulatory requirement in the pharmaceutical industry. To fulfill this requirement, a stability-indicating method (SIM) must be developed and validated to separate and quantify both the active pharmaceutical ingredient (API) and its related compounds (process impurities and degradation products). This article discusses the characteristics and relevant considerations for the development and validation of a SIM.
Concerns over the inability of a drug product (DP) to meet quality standards over the course of its shelf life have been present since the early 1970s. In 1975, the United States Pharmacopeia (USP) added a clause about the expiration dating of drug products. The US Food and Drug Administration (US FDA) provided the first stability guideline in 1984. The US FDA took further steps in 1987 by issuing guidelines for the submission of stability information and data for Investigational New Drugs (IND) and New Drug Applications (NDA). The International Conference on Harmonization (ICH) created the Q1A guideline in 1993 to harmonize the requirements for international marketing in Japan, the United States, and the European Union. Additional guidelines that followed from ICH included Q7A, GMP guide for active pharmaceutical ingredients (API). More recently, the World Health Organization (WHO) has released guidelines on the stability of pharmaceuticals (1).

Photo Credit: Getty Images/Ethan Myerson
Despite all of the requirements from the regulatory and governing entities, there is still not a clear consensus of what constitutes a stability-indicating method (SIM). The guidance documents also do not provide details about the scope and degradation study practices (1). Unfortunately, this leaves many in the industry with a requirement that must be achieved but without clear direction. This article will review existing literature and current best practices for a SIM. The objectives for a SIM, the process for development and validation, and the critical characteristics are also discussed.
Defining Objectives of the Method
The method objectives should be defined early, so the development process can be clearly established. For example, the analytes that need to be separated should be established early in the process. Stress testing or forced degradation studies can be useful in defining degradation products and the major degradative pathways. In general, for Abbreviated New Drug Application (ANDA) development of generic drug products, only compounds that exceed the ICH threshold for reporting should be investigated unless special toxicology concerns (for example, genotoxic impurities) are known. After it is known which compounds are of interest, further objectives such as desired resolution, limit of quantitation (LOQ), precision and accuracy, analysis time, and adaptability for automation can be defined (2).
For biologics, a series of methods based on orthogonal approaches may be required to achieve a SIM (1). Biologics degrade in a much different manner than small molecules. To identify all of the degradation pathways requires a variety of biochemical, biophysical, and biological methods, which can lead to a lengthy process (3). While small molecules generally degrade following first order kinetics, biologics can follow secondary or higher order kinetics that may not fit to linear or exponential curves. Degradation of biologics can be both chemical (oxidation, de-amidation, disulfide bond rearrangement, hydrolysis) and physical (aggregation, adsorption, loss of three-dimensional structure) (4).
The Process of SIM Development and Validation
There are three major steps to undergo when developing a SIM: Obtaining a suitable sample, developing the method, including the separation technique and detector, and the final validation of the method (1,5). This process was further broken down into seven steps by Bakshi and Singh (1,6). Snyder, Kirkland, and Dolan (7) and Kazakevich and Lobrutto (8) also provide extensive details on high performance liquid chromatography (HPLC) method development processes. According to the authors, the following steps must occur for a SIM:
1. Understanding of the drug substance (DS) chemistry;
2. Preliminary separation method development;
3. Defining degradation products and reference substances;
4. Method optimization;
5. Method validation.
Understanding the DS Chemistry: The focus of step 1 is on having a complete understanding of the DS active pharmaceutical ingredient (API) chemistry including physicochemical properties and anticipating its degradation products. For instance, if a new API is a derivative of one previously used and well established, it is likely that the degradation pathways will occur in a similar fashion and therefore several degradation products can be anticipated (6). Understanding the intrinsic properties of the API is very useful to determine a starting point for step 2. Several aspects of the SIM, such as diluent choice, sample preparation, separation technique (column choice), and detection technique, are dependent on the intrinsic properties of the API. Dissociation constants and partition coefficients can be used to determine an efficient extraction solvent and a proper pH for the mobile phase to provide the best separation.
The extraction solvent can be selected by matching it with the most polar functional group on the molecule. An understanding of fluorescent properties, chromatographic behavior, spectrophotometric properties, and oxidation–reduction potentials can be useful to identify the best means for measuring the analytes. A compatibility study known as a "drug-excipient interaction study" may be needed to properly assess the API and excipient interactions (2,9) for method development of a SIM for a drug product. However, it is important to note that this is merely a starting point and an API's true degradation products will not be known until forced degradation studies are conducted. For example, if the API has a nucleophilic center, it is expected to react with an electrophile to form an addition product during base degradation. However, without performing the actual base degradation study, this cannot be positively determined.
One overlooked phenomenon, especially for solid dosage forms, was discussed by Arindam and colleagues (10). A solution compatibility study should be considered in addition to the drug excipient study. This type of study evaluates solution chemistry and potential solution stability issues.
Preliminary Separation Method Development: Step 2 is related to the development of the separation process and can be the most time-consuming step. Within the pharmaceutical industry, reversed-phase LC is the most commonly used separation technique. The sample solvent, mobile-phase composition and pH, column type, and temperature can all be varied to achieve the appropriate separation. Adjustment of the mobile-phase pH can be a very good tool for the separation of ionizable compounds and is often overlooked during development (11). With advances in technology, ultrahigh-pressure liquid chromatography (UHPLC) systems equipped with small particle size columns can enable shorter run times and a faster development process without sacrificing resolution within the analysis. Software calculators to convert standard HPLC conditions to UHPLC conditions are a very useful tool if an existing HPLC method is suitable but a higher efficiency or shorter run times are desired. Sometimes it is very helpful to develop the method using UHPLC and convert it to HPLC for routine use. The development process can be performed more quickly by virtue of having shorter experiment times using the UHPLC approach. A review of the UHPLC facts and myths was recently published by Dong (12).
Dong (13) also recently discussed a three-pronged template approach to HPLC method development. This included fast isocratic LC, generic broad gradient, and multisegment gradient. For SIMs, fast isocratic methods are generally not acceptable because most are not capable of separating all components and degradation products. A generic broad gradient is commonly used for in-process monitoring in organic synthesis laboratories using a gradient such as ~5–95% B. Analytical development and quality control (QC) laboratories generally develop a custom method for each new chemical entity, which can lead to several methods each with a different column and mobile phase. A multisegment gradient is more complex but is useful when trying to separate isomers or degradation products with similar structures to that of the API. A shallow gradient may be used to maximize resolution around the API region. A steep gradient is then used to elute the strongly retained components (13).
The detectors used for liquid chromatography (LC) are UV, fluorescence, charged aerosol, evaporative light scattering, refractive index (RI), amperometry, and conductivity. While mass spectrometry (MS) detection is becoming more common, particularly for genotoxic impurities, UV detection is still the most common for SIMs. The use of diode-array detection (DAD) is advantageous to obtain spectral information (maximum absorption wavelength and peak purity) of the parent compound and each separated impurity. It should be noted that spectral differences can only be observed from compounds which show different UV spectra in the region selected. If the spectra are similar from multiple components, peak purity by DAD may not offer any additional information. An MS detector is quite useful to determine the identity of unknown compounds, which is step 3 of the process. For this reason, it is ideal to use a mobile phase that is compatible with MS (5). For a complex formulation, which contains many UV active excipients, the challenge is to minimize interferences. Detectors that are very specific, such as fluorescence and MS, can be very useful to suppress response from excipients when used properly. Alternatively, a UV wavelength program can reduce the interference as well.
Biologics may require multiple methods for demonstrating the stability-indicating profile. Assays must be able to detect changes in identity, purity, and potency (if biological activity can be defined and measured). Typical purity assay methods include electrophoresis, immune-electrophoresis, chromatography methods, and peptide mapping. These methods must be able to detect changes as a result of de-amidation, oxidation, sulphoxidation, aggregation, and fragmentation (14).
Defining Degradation Products and Reference Substances: Step 3 focuses on finding degradation products to optimize the development process (step 4). Once there is an understanding of the likely degradation products from the APIs, samples or standards are needed to determine the actual retention time of the degradation products.
The use of forced degradation studies is the primary way to obtain an adequate sample containing potential degradation products. Ideally, the DS is allowed to degrade no more than 10% using any variety of heat, acid, base, light, or oxidation. All avenues of potential degradation should be explored to ensure that all possible degradation products are being identified. Degradation above 20% is generally not acceptable as this can result in secondary degradation products that may not be seen under normal storage conditions (5). Many authors have suggested that 20% may be too much degradation and recommend the API to be degraded between 5% and 10% (2). Based on the knowledge that several assay methods have a specification of 90.0% to 110.0% over stability shelf life, a 10% target for degradation under forced degradation conditions is deemed appropriate. The anticipated assay specification and definition of a "major change of the content" from ICH Q1A should be taken into consideration when determining a proper amount of degradation. Stress testing of pharmaceuticals was thoroughly discussed by Baertschi, Alsante, and Reed (15).
Degradation products that exceed the ICH thresholds for identification or qualification encountered in stability studies require special attention, including complete identification. A number of techniques can be used for the identification of unknowns (impurities or degradation products). When the unknowns cannot be isolated in pure form, any combination of HPLC coupled with DAD, LC–MS, LC coupled to nuclear magnetic resonance (NMR), and gas chromatography (GC) coupled to MS can be used for complete structure elucidation. Singh and Rehman (16) explored the uses and limitations of each of these techniques. NMR and the synthesis of the reference compound remain the gold standard in structure confirmation of the identified impurities.
HPLC–DAD will provide the UV spectrum of an unknown as well as the peak purity of any peak (if significant in size). A similar UV spectrum to that of the API indicates that the degradant has the same chromophores as the API. A degradant with a significantly different spectrum indicates a change in the chromophoric portion of the molecules (16).
LC–MS has become a powerful tool in the identification process of unknowns in pharmaceuticals. As stated earlier, an LC–MS friendly method is ideal for a SIM. Methods that contain nonvolatile components (such as phosphate buffers) should be avoided if possible to allow for analysis by LC–MS during the unknown identification process. LC–MS is advantageous for its sensitivity, selectivity, speed, and ability to identify trace components within a complex mixture. Based on the polarity of the compounds being analyzed, different ionization techniques should be used. For nonpolar compounds, atmospheric pressure photo ionization (APPI) is the best technique. Electrospray ionization (ESI) can be used for compounds with low polarity to ionic molecules. Atmospheric pressure chemical ionization (APCI) should be used for compounds with weak to medium polarity. Matrix-assisted laser desorption–ionization (MALDI) is primarily used for large biomolecules or biopolymers such as proteins and peptides. Atmospheric pressure matrix-assisted laser desorption–ionization (AP–MALDI) is similar to MALDI, but performed at atmospheric pressure and can be used to ionize large molecules (16). Deuterium-exchanged LC–MS has been shown to be very useful for the identification of isobaric compounds (for example, N-oxides and hydroxides). Cation-enhanced MS is very helpful to increase ionization efficiency for some molecules using electrospray ionization, for example, vitamin D derivatives (17–19). For structure elucidation, use of exact mass spectrometers such as time-of-flight (TOF)-MS systems and sequential multistage fragmentation using ion trap systems is becoming indispensable.
Along with LC–MS, LC–NMR has also become a technique of choice for some challenging identification of unknowns. LC–NMR cannot match the sensitivity of LC–MS but can definitively provide a molecular structure when LC–MS data is insufficient or isolation of the unknown is not possible. As with LC–MS, the LC method must be compatible with this LC–NMR technique. Protons from the mobile-phase components can cause interference. For this reason, deuterated solvents should be used (16).
Combinations of techniques such as tandem mass spectrometry (MS-MS) or LC–NMR–MS are additional tools that can efficiently provide important information about unknown compounds.
While the majority of unknown identifications are performed by any of the described LC techniques above, some compounds (such as raw material and critical reagents) may be better suited for GC analysis. This may be a result of the volatile nature of the molecule or its lack of polarity making it unsuitable for LC separation or detection. In these cases, GC–MS may be an appropriate method. Electron ionization (EI) and chemical ionization (CI) are two techniques used. EI is an aggressive ionization technique that may not produce a molecular ion, thereby making identification difficult. For this reason CI, which is a soft ionization technique, is preferred (16).
Method Optimization: During initial method development, it is recommended to make significant changes in the separation variables to create significant changes in the chromatographic profile, so step 4 can be carried out quickly as needed. Smaller adjustments can be made after the significant separation requirements have been achieved (5). The fine-tuning step or method optimization often involves changes to the mobile phase (pH, buffer type and ionic strength, pH), gradient profile (gradient time, number of segments), flow rate, injection volume, and sample concentration to ensure that the method can separate all impurities and degradants and is compliant to ICH guidelines in sensitivity and other requirements.
The final stage of step 4 should be a method prequalification to ensure that the newly developed method is "validatable." Several of the experiments that are to be performed during validation should be completed at this point to ensure the method is fully capable of meeting the validation requirements. Since method validation is a good manufacturing practice (GMP) process and method development is not, this is an important step to ensure that the validation step proceeds smoothly without unexpected failures or anomalies (13).
Method Validation: The final step of the SIM process is validation of the finalized method. The ICH guidelines (Q2A, Q2B) provide detailed validation requirements (20). Validation should include specificity, linearity, accuracy, precision, intermediate precision, LOQ, and robustness. If a thorough and complete development process has already occurred, the validation step is merely a formality of repeating and documenting several experiments that should have already occurred. To establish specificity of a SIM, it is critical to show separation of all known key components, including process impurities and degradation products, and to demonstrate peak purity on the APIs when possible. However, it is important to note that while peak purity is a useful tool for large peaks, the same cannot be said for peaks at or near the LOQ of the method. At these low levels, the baseline noise can interfere with peak purity calculations and data is unreliable at best. An example of a typical method validation process, except specificity, is given in Figure 1.
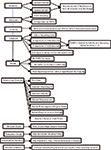
Figure 1: Typical validation characteristics of a method. Note: Please see Figure 2 for specificity.
This is a more general approach. Phase-specific strategies are normally performed by different companies for method development and validation, especially for ANDA development. For generic drug product development, the time line is condensed and attention is given to the similarity profile of the drug in development with the commercial innovative drug.
Recently, the USP Validation and Verification Expert Panel published a stimuli article on life cycle management of analytical methods. According to this paper, instead of current discreet steps of development, validation, or verification, life cycle management of analytical methods should be based on ICH Q8, Q9, and Q10. Analytical target profiles, quality by design concepts, control strategies, knowledge management, and risk-based approaches are the keys to this new concept for life cycle management. It also emphasizes an understanding of the variability in the data, probability, and confidence interval. As a result of these recommendations, the Validation and Verification Expert Panel proposed that the concepts addressed in USP 1225, 1226, and 1224 be revised to integrate the processes for demonstrating that an analytical procedure is fit for purpose throughout its life cycle, and that these three chapters be compiled into a single general information chapter 1220 and a new general chapter 220 that specifies the basic requirements (21).
SIM Characteristics
The primary characteristic of a SIM is its ability to separate degradation products or impurities from the actives and each other (when needed). The stability indicating nature can be established by the following approach, as shown in Figure 2.
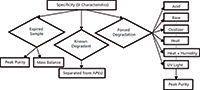
Figure 2: Characteristics of the stability-indicating nature of a method.
Forced Degradation: During forced degradation, the most likely degradation products are generated. These degradation products must be separated from each other and from the active ingredient. Generally, peak purity is demonstrated by using a diode-array detector for the homogeneity of a chromatographic peak when using UV detection.
When structurally similar compounds with very similar UV spectra are expected, mass spectral peak purity can be obtained for the separations that use MS-compatible mobile phases.
If a different detection technique is used, an orthogonal separation or a stretch gradient is appropriate to show peak homogeneity. A special case in this category would be a drug product where multiple drug substances are present. In those cases, a forced degradation study should be done at a minimum with each DS, placebo, and the drug product. A design of experiment study may be undertaken to understand the inter API reaction chemistry. It is important to note that when a new impurity is observed during a stability study, retention time for that impurity should be examined for all the methods to demonstrate specificity. Impurity detected by the assay method may be examined but not necessarily be investigated if a corresponding unknown is seen by the impurity method.
Expired Samples: The purpose of the forced degradation is to obtain the most likely degradation products. For a mature product, if during the life cycle management process a gap is identified regarding the stability-indicating nature of the method, a complete forced degradation study is not necessary in most cases.
Expired samples from different lots may be used instead of forced degradation. The same strategy of peak purity, orthogonal separation, or stretch gradient approach can be utilized for the confirmation of separation of the degradation products using expired sample when compared to release data.
Normally separation of known degradation products by itself does not constitute a SIM, unless forced degradation or expired sample analysis is performed.
Table I shows generic forced degradation conditions for a SIM. As stated above, the appropriate stress concentrations and times should be determined for each product or API to achieve approximately, but not more than, 10% degradation. Some APIs may be unresponsive to some of the forced degradation conditions. This is acceptable in these cases so long as due diligence is shown.

Table I: Mass balance calculation from typical forced degradation conditions or expired sample analysis
Mass Balance Approach: Mass balance is another tool that can be used to demonstrate the stability indicating nature of SIMs in the absence of forced degradation data. The idea is that for any loss observed in the API over time, a corresponding increase in the degradation products should also be observed. This approach can be used on either expired samples or forced degradation samples as long as the "initial" (that is, release or time zero) assay and degradation results are available for comparison. The assay loss (final–initial) in weight (%) is added to the degradation increase (final–initial) to obtain a mass balance percentage. A negative value indicates more API loss; a positive value indicates more degradation; and a value of 0.0% would be a perfect correlation.
Analysis of Recombinant Protein Therapeutics
There are many aspects to consider when evaluating biologics. Absolute purity is very difficult to determine because of heterogeneity. Shelf life of a biologic has to be supported by long-term, real-time, and conditional stability data. Degraded product may be active but may present issues because of immunogenicity or bioavailability (14).
No single assay or parameter is sufficient to evaluate a biological material. Stability for conjugated or combination products may need a surrogate test (versus in vitro or in vivo potency or efficacy tests). Potency of a biologic must be shown if biological activity can be defined and measured. In this case, results are expressed relative to a reference material. Dissociation of conjugated products should be monitored in real-time or temperature studies. Purity is typically assessed using multiple methods (14).
For antibody-drug conjugates (ADC) an enzyme-linked immunosorbent assay (ELISA) and cell-based assay should be in place early in the development. ADCs should also have a test for heterogeneity to ensure product consistency. Testing may involve measuring aggregates and fragments, charge variants, unconjugated monoclonal antibodies, average drug to antibody ratio, and drug distribution. The most effective techniques for measuring heterogeneity are hydrophobic-interaction LC (HIC–LC), size-exclusion chromatography (SEC–HPLC), reversed-phase HPLC, capillary electrophoresis with sodium dodecyl sulfate (CE–SDS), MS, and peptide mapping (22).
Examples of tests for structural characterization and confirmation (with typical techniques) include (23):
- Amino acid composition: Various hydrolytic and analytical procedures;
- Terminal amino acid sequence: Appropriate analytical procedure;
- Peptide map: HPLC;
- Sulphydryl groups and disulfide bridges: Peptide mapping, mass spectrometry;
- Amino acid sequence: Determined by techniques above and compared with sequence of amino acids deduced from the gene sequence of the desired product;
- Carbohydrate structure: Neutral sugars, amino sugars, and sialic acids should be determined.
Physicochemical property tests (with typical techniques) may consist of the following (23):
- Molecular weight or size: Size-exclusion chromatography, SDS-polyacrylamide gel electrophoresis, mass spectrometry, or other appropriate techniques;
- Isoform pattern: Isoelectric focusing;
- Extinction coefficient: UV–vis spectrophotometry;
- Electrophoretic patterns: Polyacrylamide gel electrophoresis, isoelectric focusing, SDS-polyacrylamide gel electrophoresis, Western-blot, capillary electrophoresis;
- LC patterns: Size exclusion, reversed phase, ion exchange, affinity;
- Spectroscopic profiles: Circular dichroism, NMR.
Impurities or degradation tests (with typical techniques) consist of (23):
- Truncated forms: HPLC, SDS-PAGE;
- Other modified forms: HPLC, MS, CE, circular dichroism;
- Aggregates: CE, size-exclusion chromatography.
Remediation: Risk-Based Approaches
Often within QC laboratories, assay or degradation methods may be several years old. It is likely that the validation documents do not contain sufficient data to support the stability-indicating nature of the method. This does not necessarily mean that the methods are not stability indicating. In these cases, forced degradation or expired sample analysis can be conducted to remediate this gap. However, these studies can take time and there may be a concern over current marketed product. Alternatively, a risk-based approach can be taken in these cases by reviewing stability assay and degradation data. Methods that demonstrate a mass balance within 2.0% can be classified as low risk. These methods are most likely stability indicating as evidenced by the correlation of assay and degradation data. Studies that exhibit a mass balance between 2.0% to 5.0% are moderate risk and may not need immediate attention. Studies that show a mass balance above 5.0% are high risk and these products or methods should receive higher scrutiny.
Conclusion
Current practices and considerations for a stability-indicating method were reviewed in this article. Recommended processes and best practices for method development, validation, and other characteristics for a SIM were discussed from a practicing pharmaceutical scientist's perspective. New USP initiatives on life cycle management of analytical procedures provide conceptual changes of the method validation, verification, and transfer approaches.
Acknowledgments
The input from various colleagues in the pharmaceutical industry at large is acknowledged, particularly the members of the analytical services team at Novartis. Many helpful discussions, encouragement, and support provided by Mr. Maurice Shokouhi are also acknowledged.
Disclaimer
The views provided in this article are from the authors only and not from Novartis or any of its affiliates.
References
(1)R.M. Maggio, S.E. Vignaduzzo, and T.S. Kaufman, TrAC, Trends Anal. Chem. 49, 57–70 (2013).
(2)B.P. Shah, S. Jain, K.K. Prajapati, and N.Y. Mansuri, Int. J. Pharm. Sci. Res. 3(9), 2978–2988 (2012).
(3)G. Dutton, Genet. Eng. Biotechnol. News 27(18), (2007).
(4)S.G. Magil, Design and Interpretation of Accelerated Stability Studies of Biologics, Cambridge Healthtech Institute's Fourth Annual The Bioprocessing Summit 2012 (20–23 August 2012); www.bptc.com
(5) J.W. Dolan, LCGC North Am. 20(4), 346–349 (2002).
(6) M. Bakshi and S. Singh, J. Pharm. Biomed. Anal. 28(6), 1011–1040 (2002).
(7) L.R. Snyder, J.J. Kirkland, and J.W. Dolan, Introduction to Modern Liquid Chromatography, 3rd Ed. (John Wiley & Sons, Hoboken, New Jersey, 2010).
(8) Y.V. Kazakevich and R. LoBrutto, HPLC for Pharmaceutical Scientists (John Wiley & Sons, Hoboken, New Jersey, 2007).
(9) K. Huynh-Ba, in Handbook of Stability Testing in Pharmaceutical Development (Springer, 2009), p. 153.
(10) A. Roy et al., "LC Method Development for Drug Products and Impurity Analysis," paper presented at Pittcon, Chicago, Illinois, 2009.
(11) M. Swartz and I. Krull, LCGC North Am. 23(6), 589–590 (2005).
(12) M. Dong, LCGC Europe 26(11), 637–645 (2013).
(13) M. Dong, LCGC North Am. 31(8), 612–621 (2013).
(14) J. Linder, Stability of Biological Products. http://www.raci.org.au/document/item/884. (August 21, 2012).
(15) S.W. Baertschi, K.M. Alsante, and R.A. Reed, Pharmaceutical Stress Testing: Predicting Drug Degradation 2nd Ed. (Informa Healthcare, 2011).
(16) R. Singh and Z. Rehman, J. Pharm. Educ. Res. 3(1), 54–63 (2012).
(17) A. Roy et al., Current Trends in Mass Spectrometry supplement to Spectroscopy, LCGC North Am., and LCGC Europe, (October, 2011).
(18) A. Roy et al., Chemistry Today 27(2), (2009).
(19) A. Roy et al., "Impurity Analysis in Pharmaceuticals," paper presented at Pittcon, Orlando, Florida, 2010.
(20) www.ich.org, accessed on 2 January 2014.
(21)
, accessed on 30 December 2013.
(22) J. Harris, F. Jacobson, C. Jochheim, G. Amphlett, K. Francissen, and L. McLeod, BioProcess Int. 9(8), 12–22 (2011).
(23) European Medicines Agency (EMEA), Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products (1999) http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002824.pdf.
Arindam Roy is currently an associate director of Quality Control at Alkermes, Inc., USA. When the article was written, he was with Novartis as an associate director of analytical services. He holds a PhD in analytical chemistry from the University of Missouri-Rolla, in Rolla, Missouri. He is regarded as a leader in analytical development and quality control of pharmaceuticals.
Anthony Wilken is a specialist in the analytical services laboratory at Novartis OTC. He holds a bachelor's degree in both chemistry and mathematics from Hastings College, in Hastings, Nebraska. He has more than 12 years of experience in pharmaceutical analysis.
Joseph Henry is a director of analytical science and technology at Novartis OTC, USA. He holds an MS in analytical chemistry from the Ohio State University, in Columbus, Ohio. He has extensive experience in the area of pharmaceutical analytical development and quality control.
Luis R. Collazo Malave is a director of quality control at Novartis OTC, USA. He holds a PhD in organic chemistry from the New Mexico State University, in Las Cruces, New Mexico. He has extensive experience in the area of pharmaceutical quality control and quality assurance.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Quantifying Terpenes in Hydrodistilled Cannabis sativa Essential Oil with GC-MS
April 21st 2025A recent study conducted at the University of Georgia, (Athens, Georgia) presented a validated method for quantifying 18 terpenes in Cannabis sativa essential oil, extracted via hydrodistillation. The method, utilizing gas chromatography–mass spectrometry (GC–MS) with selected ion monitoring (SIM), includes using internal standards (n-tridecane and octadecane) for accurate analysis, with key validation parameters—such as specificity, accuracy, precision, and detection limits—thoroughly assessed. LCGC International spoke to Noelle Joy of the University of Georgia, corresponding author of this paper discussing the method, about its creation and benefits it offers the analytical community.












