Waters - Size-Exclusion Chromatography for the Impurity Analysis of Adeno-Associated Virus Serotypes
Monitoring the size heterogeneity of AAV-based gene therapy therapeutics is potentially important to ensure consistent product quality and efficacy. We demonstrate that the levels of both high-molecular weight (HMW) and low-molecular weight (LMW) impurities in AAV capsid preparations can be separated on a Waters 450 Å Protein BEH SEC column for a series of AAV serotypes.
As the development of gene therapy products accelerates, the need to develop sound and efficient analytical strategies to help guide the development of manufacturing processes and evaluate the quality of clinical adeno-associated virus-(AAV)–based gene therapy materials has become more important. Among other critical quality attributes, the levels of potential AAV high molecular weight (HMW) species and AAV fragments or low molecular weight (LMW) species may also require monitoring (1). Here we present optimized non-denaturing size-exclusion chromatography (SEC) methods that can separate soluble AAV for several AAV-CMV-GFP serotypes including AAV1, AAV2, AAV5, AAV6, AAV8, and AAV9.
Experimental Conditions
AAV sample: AAV serotypes ranging from 1.6 × 1012 to 6.7 × 1013 GC/mL
LC system: ACQUITYTM UPLCTM H-Class Bio
Sample temp.: 6 ̊C
Column temp.: 25 ̊C
Injection volume: 3 μL
Column: XBridgeTM Protein BEH SEC, 450 Å, 3.5 μm, 7.8 × 300 mm
Fluorescence detector: Excitation: 280 nm; Emission: 350 nm
Mobile phase: 10 mM NaH2PO4, 10 mM Na2HPO4, 200–400 m KCl, pH 6.6 (HCl)
Flow rate: 0.6 mL/min
Results and Discussion
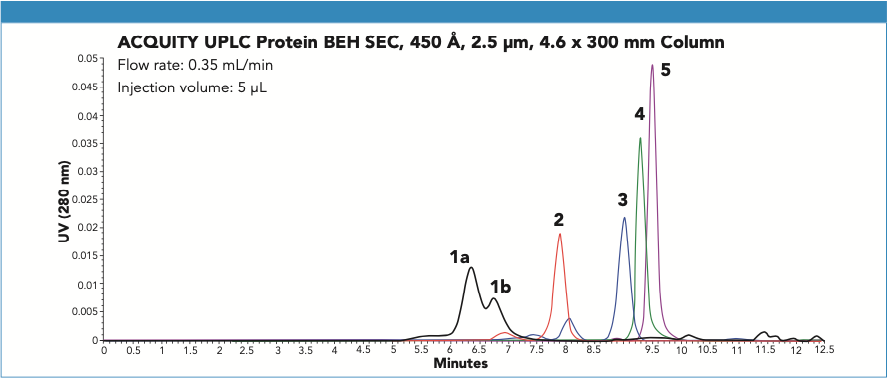
The upper analyte size limitation for SEC separations of proteins is approximately 100–200 nm (subvisible) depending on SEC particle size. Above these limits, the analyte may be altered due to shear forces or trapped by the frits or packed bed of the column. Therefore, these subvisible entities are analyzed using dynamic light scattering and nanoparticle tracking analysis (NTA) methods, among others (2). With these considerations, the SEC separation of soluble AAV monomer, and HMW, and LMW was evaluated on a 450 Å pore-size BEH diol-bonded SEC column. This column was previously demonstrated to be effective in the separation of IgM pentamer and dipentamer in Waters laboratories (Figure 1) and has a reported diameter of 35 nm (3). AAV has a total protein and ssDNA molecular weight of 5500 kDa; however, the compact structure has a diameter of only 25 nm (4). Therefore, it was predicted that these high-efficiency SEC particles could provide the accessible pore volume needed. A 3.5 μm particle size was selected over 2.5 μm particles to reduce potential sample sieving and shearing effects. Intrinsic protein fluorescence detection was also employed to provide maximum optical sensitivity.
Figure 1: Shown is the SEC separation of several protein structures. Compounds are: 1a. IgM dipentamer (1.8 kDa), 1b. IgM pentamer (900 kDa), 2. thyroglobulin (667 kDa), 3. apoferritin (443 kDa), 4. β-amylase (200 kDa), 5. IgG (150 kDa). UV absorbance (280 nm) and the identities of the peaks observed for the IgM sample were confirmed by SEC–MALS analysis.
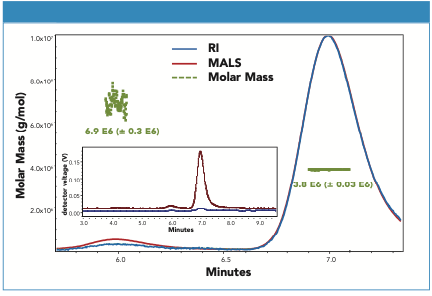
The SEC separation of a null control sample (AAV without ssDNA) was evaluated using a Wyatt microDAWN Multi-Angle Light Scattering (MALS) detector and a Waters ACQUITY Refractive Index (RI) detector (Figure 2). The SEC–MALS data confirmed that adequate separation between the dimeric and monomeric AAV forms was observed. Putative multimeric forms preceding dimer were also observed by fluorescence but could not be assigned molecular weights due to their low abundance.
Figure 2: SEC-MALS of AAV8-null sample using refractive index (RI) for concentration measurement are shown. The MALS (red) and RI (blue) signals are normalized and the average and distribution of determined molar masses (green) were determined using Wyatt Astra (v. 7.3.1.9) based on a dn/dc of 0.185 and using a “sphere” model for the icosahedral AAV.
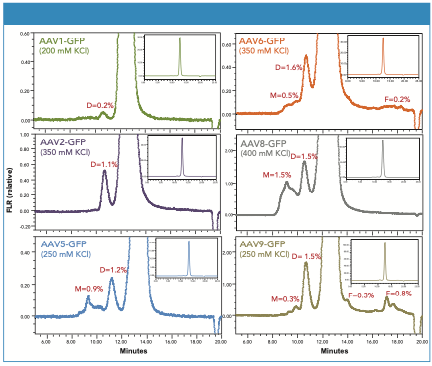
A mobile-phase consisting of 20 mM sodium phosphate, pH 6.6, with varying amounts of KCl (200–400 mM) was found to maximize the recovery of dimer and multimer for serotypes AAV1, AAV2, AAV5, AAV6, AAV8, and AAV9 (Figure 3). We observe that the peak shapes for both the dimer HMW species and monomeric AAV forms are symmetrical and return to baseline appropriately. In addition, detectable levels of multimeric AAV forms were observed for several of the serotypes. Significant amounts of LMW forms were only observed in AAV9 and AAV6.
Figure 3: Shown are the SEC separations of a series of AAV serotype control samples containing ssDNA coding for green fluorescent protein (GFP). The peak percentages for dimer (D), multimer (M), and fragments (F) are provided. The chromatogram baselines are zoomed approximately 50x versus the full-scale chromatogram shown in the inset.
Conclusions
A BEH SEC column with an average pore size of 450 Å and a particle diameter of 3.5 μm was demonstrated to be effective in the separation of AAV monomers from their HMW dimers, lower valency multimers, and LMW fragments. The minimal amount of ionic strength (KCl) required for optimal peak shape and recovery varied by serotype, and separations are presented for serotypes AAV1, AAV2, AAV5, AAV6, AAV8, and AAV9.
References
(1) J. F. Wright, Gene Therapy 15(11), 840–8 (2008).
(2) (a) J. F. Carpenter, T. W. Randolph, W. Jiskoot, D. J. Crommelin, C. R. Middaugh, G. Winter, Y. X. Fan, S. Kirshner, D. Verthelyi, S. Kozlowski, K. A. Clouse, P. G. Swann, A. Rosenberg, and B. Cherney, J Pharm Sci 98(4), 1201–5 (2009); (b) B. Slutter, and W. Jiskoot, Expert Opin. Drug Delivery 13(2), 167–70 (2016).
(3) R. Saber, S. Sarkar, P. Gill, B. Nazari, and F. Faridani, Sci. Iran. 18(6), 1643–1646 (2011).
(4) S. Vernon, J. Stasny, A. Neurath, and B. Rubin, J. Gen. Virol. 10(3), 267–272 (1971).
Waters, The Science of What’s Possible, ACQUITY, UPLC, and XBridge are trademarks of Waters Corporation. All other trademarks are the property of their respective owners.

Waters Corporation
34 Maple Street, Milford, MA 01757
tel. 508 478 2000
Website: www.waters.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)