Showa Denko - HILIC Columns for Phosphorylated Sugar Analysis
Various types of phosphorylated saccharides are involved with glycolysis, glyconeogenesis, glycometabolism, and other naturally occurring biological phenomena. One example of a phosphorylated saccharide includes fructose-2,6-bisphosphate, the most powerful activator of phosphofructokinase. Recent research has shown the phosphorylated saccharide intermediates created during glyconeogenesis and glycometabolism can be used in other ways than naturally intended for a number of applications, drastically broadening the interest in phosphorylated saccharide.
Phosphorylated saccharides occupy a central position in carbohydrate metabolism. Numerous sugar interconversions occur when the sugars are linked to phosphates and di-phosphates. When this occurs, the phosphorylated saccharides can function as glycosyl donors in many transglycosylation reactions, providing a variety of oligosaccharides, glycosides, and polysaccharides.
Phosphorylated sucrose and fructose have been synthesized by the transglycosylation process in plants, glycogen in animal tissues, and chitin. The isolated phosphorylated sucrose has further shown to potentially be effective in experimental drugs. How the new drugs interact within the body is directly affected by which carbon contains the phosphate group. Phosphorylated fructose has been recently suggested to be a part of the creation of ATP.
Phosphorylated mannose and glucose were found to be potent inhibitors of lysosomal hydrolase endocytose by fibroblasts, plants, and bacteria. It was recently proposed that phosphorylated mannose is an essential component of the recognition marker on acid hydrolases. This was direct evidence from the phosphorylated mannose’s ability to uptake forms of lysosomal enzymes.
With the knowledge of these few applications, the field has been exponentially growing.
Four variations of phosphorylate saccharides were analyzed by a Shodex HILICpak VT-50 2D column under LC–MS conditions. The sample contained two phosphorylated glucose and two phosphorylated fructose, each phosphorylated at a different carbon, providing different biological functions. This analytical condition can also be used with other detectors including reflective index (RI), evaporative light scattering detector (ELSD), and corona charged aerosol detector (CAD).
Experimental Conditions
The analysis of glucose-6-phosphate, fructose-6-phosphate, glucose- 1-phosphate, and fructose-1-phosphate was accomplished using the Shodex HILICpak VT-50 2D (2.0 mm ID × 150 mm ID, 5 μ), a HILIC column suitable for LC–MS. The column temperature was 60 oC and the flow rate was 0.3 mL/min. The eluent conditions were 25 mM HCOONH4 aq./CH3CN = 80/20. An injection volume of 5 μL of 1 μM of each sugar was used for the experiment. The Column: HILICpak VT-50 2D, Column temperature: 60 oC, Injection volume: 5 μL, Eluent: 25 mM HCOONH4 aq./CH3CN : 80/20, Flow rate: 0.3 mL/min. Detector: ESI–MS (SIM Negative: m/z 259). Sample: 1-and 6- phosphorylated saccharides.
HPLC system was coupled with an ESI–MS (SIM negative: m/z 259) detector.
Results
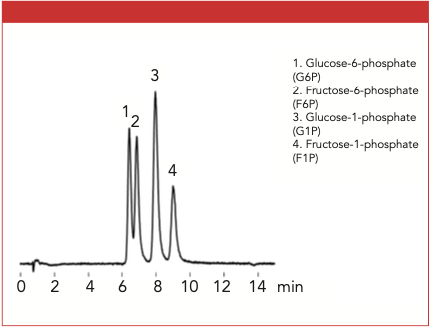
The aqueous sample containing the four phosphorylated sugars was analyzed successfully using HILIC and MS detection with the Shodex HILICpak VT-50 2D column (Figure 1). From this single run, each phosphorylated saccharide was prominently separated. The closest peaks were glucose-6-phosphate and fructose- 6-phosphate, both eluted between 6 and 7 min.
Figure 1: The analysis of phosphorylated saccharides using the Shodex VT-50 2D column.

ShodexTM/Showa Denko America, Inc.
420 Lexington Avenue Suite 2335A, New York, NY, 10170
tel. (212) 370-0033 x 109
Website: www.shodexhplc.com


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)