Single Calibrant Approach for the Analysis of Unknowns Using Dual Gradient Pump and Charged Aerosol Detection
The Application Notebook
Quantification of substances, such as drug impurities or library compounds when pure standards are not available is difficult yet often necessary.
Chris Crafts, Bruce Bailey and Ian Acworth, Dionex Corporation, Sunnyvale, California, USA.
Quantification of substances, such as drug impurities or library compounds when pure standards are not available is difficult yet often necessary. This is often accomplished by HPLC based on relative response using low wavelength UV detection. The dependence of response on the optical properties of each component can lead to large errors in estimated quantity. The Dionex Corona Charged Aerosol Detector (CAD) is a mass sensitive detector that has been shown to provide near uniform response for nonvolatile analytes provided that the eluent remains constant.1 Response, however, can change during gradient conditions. An effective way to normalize response is to use an inverse gradient post-column addition.2 The Dionex UltiMate 3000 RSLC system equipped with dual gradient pump (DGP) greatly facilitates the use of two simultaneous gradient profiles on one HPLC/UHPLC platform. This application note illustrates the use of CAD with this DGP system for single calibrant estimation of unknown substances.
Materials and Methods
A Dionex UltiMate 3000 RSLC system equipped with a DGP- 3600RS pump, with a Corona ultra CAD (Figure 1).
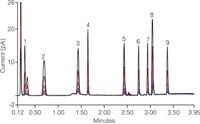
Figure 1: Overlay of five injections of test mix at each of the five concentration levels from 11â170 ng on-column using Corona ultra detection with inverse-gradient.
HPLC Parameters:
Column: Acclaim RSLC 120 C18 3 µm 120 Å, 3.0 × 33 mm
Column temperature: 30 °C
Mobile phase A: 10 mM ammonium acetate, pH = 4.5
Mobile phase B: Acetonitrile
Flow rate: 1.0 mL/min each pump (2 mL/min to detector)
Injection volume: 2 µL
Corona ultra: Nebulizer temperature: 25 °C; filter: high
A group of nine chemically diverse compounds were dissolved and then combined to give a mixture of equal mass concentration for each analyte.
Results
Five replicate injections were made at each of 5 concentration levels from 11 to 170 ng on column. The correlation coefficients for all nine linear fit curves were ≥ 0.999. Back calculations using the nine curves at each calibrant's 20 ng on column level yielded 81 data points of which 89% fell within 50% of their expected values. If the data related to one outlier (SDS) is removed, then 98% of the results fall within 50% of their expected values. UV data for the same sample set does not allow for these calculations across the entire set. Only five peaks are detected at 254 nm and six peaks at 210 nm with an overall response deviation between analytes, which is five to six times higher then that observed on the CAD.
Conclusion
Quantification of substances in the absence of pure standards is necessary in many stages of the pharmaceutical process. A single UltiMate 3000 DGP-RSLC platform with Corona ultra detection provides a simple means of obtaining better uniformity of response than low wavelength UV for nonvolatile analytes. This improves the estimation of unknowns without the need for analytical standards for all components. The sensitivity and quantitative accuracy provided by this approach may be useful for many applications such as analysis of impurities and degradants, monitoring compound synthesis and quality of library compounds, cleaning validation and mass balance experiments.
References
1. Dependence of Response on Chemical Structure, Application Brief 70-8913.
2. T. Gorecki et al., Anal. Chem., 78(9), 3186–3192 (2006).
Corona, UltiMate, CAD and Acclaim are registered trademarks and ultra is a trademark of Dionex Corporation.

Dionex Corporation
1228 Titan Way, PO Box 3603, Sunnyvale, California 94088, USA
tel. +1 408 737 0700 fax +1 408 730 9403
Websites: www.coronaultra.com, www.dionex.com

A Guide to (U)HPLC Column Selection for Protein Analysis
April 16th 2025Analytical scientists are faced with the task of finding the right column from an almost unmanageable range of products. This paper focuses on columns that enable protein analysis under native conditions through size exclusion, hydrophobic interaction, and ion exchange chromatography. It will highlight the different column characteristics—pore size, particle size, base matrices, column dimensions, ligands—and which questions will help decide which columns to use.
The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.














