Rapid Analysis of Pesticides in Difficult Matrices Using GC–MS–MS
The Application Notebook
Pesticides are widely used in agriculture to protect crops and to improve efficiency of production. Consequently, governments, food producers and food retailers have a duty to ensure that any residues occurring in foods for human consumption are at or below statutory maximum residue levels (MRLs). Regulation EC 396/2005 adopted in the European Union sets MRLs for more than 500 different pesticides in over 300 different food commodities.
Introduction
Pesticides are widely used in agriculture to protect crops and to improve efficiency of production. Consequently, governments, food producers and food retailers have a duty to ensure that any residues occurring in foods for human consumption are at or below statutory maximum residue levels (MRLs). Regulation EC 396/2005 adopted in the European Union sets MRLs for more than 500 different pesticides in over 300 different food commodities.1 Many of these MRLs are set at a default value of 0.01 mg/kg, the typical limit of determination of routine analytical methods. Thus, there is a requirement for residue laboratories to test a wide array of foods for a large number of pesticides at concentrations at or below 0.01 mg/kg, with low costs and fast turnaround. This is most often achieved using multiresidue methods based on the use of a combination of LC–MS–MS and GC–MS techniques to determine pesticide residues in a single generic solvent extract of the sample. One such example is the quick, easy, cheap, effective, rugged and safe (QuEChERS) procedure, which is based on acetonitrile extraction and dispersive solid-phase extraction.2 Because acetonitrile is readily compatible with LC–MS–MS and allows analysis of several hundred pesticides this approach has gained in popularity. Many of the less polar semi-volatile pesticides not amenable to LC–MS can be analysed using GC–MS. Unfortunately, the analysis of acetonitrile QuEChERS extracts by GC–MS is more problematic, primarily due to degradation of the GC-column phase by the polar solvent, vapour overload of the insert liner due to the high thermal expansion coefficient, contamination of the system by co-extractives and low crop concentration in the final extracts.
Because of these problems, the detection limits for some pesticides can be too high to meet MRL requirements. It is a common practice to overcome these difficulties by concentration of the extract by evaporation, exchanging acetonitrile to a more appropriate GC solvent, or by using large volume injection techniques. However, there is the potential to lose volatile analytes during evaporation and solvent exchange. Also the use of large volume injections can lead to more rapid contamination of the injection inlet as well as degradation of the analytical column. An alternative approach is to make use of the high sensitivity and selectivity of the Thermo Scientific TSQ Quantum XLS GC–MS–MS instrument, which can achieve the 0.01 mg/kg target reporting limits even with relatively low volume injections. This also overcomes the problems associated with the thermal expansion of acetonitrile and reduces the amount of matrix injected. This application note describes the analytical methodology for a fast multiresidue pesticide determination in a difficult matrix (fruit jam), using the QuEChERS extraction/clean-up procedure in combination with the TSQ Quantum XLS GC–MS–MS system as the detection system.

Figure 1: Extracted ion chromatogram of quantifier (upper trace) and qualifier ions (lower trace) for (a) dichlofluanid, (b) deltamethrin in a fruit preserve sample spiked at 0.01 mg kg-1 and (c) captan at 0.02 mg kg-1.
Experimental Conditions/Methods
Sample preparation
The strawberry jam samples were extracted using the citrate buffered QuEChERS procedure. Homogenized sample (10 g) was mixed with water (10 mL) and acetonitrile (10 mL). After the addition of internal standard (triphenylphosphate, TPP) the mixture was shaken for 1 minute. Then MgSO4 (4 g), NaCl (1 g), disodium hydrogen citrate (0.5 g) and trisodium citrate (1 g) were added and the mixture shaken for 1 minute and then centrifuged for 5 minutes at 3000 U/min. An aliquot of the acetonitrile portion (2 mL) was transferred to a new tube and MgSO4 (300 mg) and primary-secondary amine (PSA) sorbent (50 mg) added. The mixture was shaken (1 minute) and centrifuged for 5 minutes at 3000 U/min. An aliquot of the supernatant (1 mL) was immediately transferred into a GC vial and acidified (10 μL of 5% formic acid in acetonitrile). Then 1.0 μL of extract was injected into the GC–MS–MS system. The final concentration of sample was 1 g/mL of extract.
Instrument set-up and conditions
Determination of pesticides was performed using a TSQ Quantum XLS GC–MS–MS system, equipped with a split/splitless injector and Thermo Scientific TriPlus automatic liquid sampler. The analytical column used was a Thermo Scientific TR-Pesticide, 30 m × 0.25 mm i.d., 0.25 μm film thickness.
Results and Discussion
The samples of fruit jam were extracted with acetonitrile followed by dispersive SPE clean-up with PSA prior to detection by GC–MS–MS. The high sensitivity and selectivity of the TSQ Quantum XLS GC–MS–MS system has enabled direct splitless analysis using low volume (1.0 μL) aliquots of acetonitrile extracts. This has significantly simplified the sample preparation procedure, while meeting method performance criteria specified by EU method validation and quality control procedures for pesticide residues in food and feed.4 To overcome matrix effects, calibration of the GC–MS–MS system was performed using matrix-matched standard calibration solutions.5,6
Validation of the methodology was performed using samples spiked with known amounts of selected pesticides at concentrations between 0.01 and 0.05 mg/kg for 89 analytes. A further 7 analytes were spiked at higher levels, as reporting limits for these pesticides are correspondingly higher in UK monitoring. Except for chlorothalonil, all of the pesticides met the EU DG SANCO method validation criteria.4 Pesticides such as captan, dichlofluanid and iprodione, which are frequently difficult to analyse, showed good recovery and precision data refer to Table 1 with troublesome compounds. The study on the improvement of chlorothalonil recovery from the matrix is now under further investigation. In all cases the detection limits comply with required MRLs.
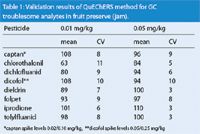
Table 1: Validation results of QuEChERS method for GC troublesome analytes in fruit preserve (jam).
Conclusion
The QuEChERS-GC–MS–MS multiresidue method described here is a simple, rapid and accurate approach suitable for the monitoring of GC amenable pesticides in accordance with EU requirements. Another advantage of the extraction method used is its applicability to pesticides amenable to LC analysis. During method validation it was found that recovery, % CV and linearity data were within EU DG SANCO criteria for all 96 pesticides, except chlorothalonil at 0.01 mg/kg and dicofol. However the recovery data for chlorothalonil spiked at 0.01 mg/kg (mean 63%, 11% CV) and the consistent recovery and precision data obtained for dicofol showed that the methodology was suitable for screening purposes. Extracted ion chromatograms of SRM traces of analytes demonstrated excellent selectivity with no interferences observed and excellent signal/noise ratios (> 5:1), for all analytes at the lowest calibrated level (typically 0.005 mg/kg). The robustness of the system was further demonstrated during validation and analysis of pork ham samples for the UK Monitoring Programme, when similarly good quality analytical data was obtained for these analytes.
References and Acknowledgements
1. REGULATION (EC) No 396/2005 and amendments, Feb. 23 2005
2. M. Anastassiades et al., J. AOAC Int., 86(2), 412 (2003).
4. European Commission, Doc No. SANCO/10684/2009
5. M. Godula et al, J. High Resol. Chromatogr., 22(7), 395–402 (1999).
6. J. Hajšlová et al, J. Chromatogr. A, 800, 283–295 (1998).
The study was perfoemd under collaboration between Thermo Fisher Scientifics' Food Safety Group and Food and Environmental Research Agency, York, UK.

Thermo Fisher Scientific Inc.
2215 Grand Avenue Parkway, Austin, Texas, USA
tel. +1 800 532 4752 fax +1 561 688 8731
Website: www.thermofisher.com















