Simultaneous Determination of Formononetin, Biochanin A, Daidzen and Genistein in Trifolium pratense (Red Clover) by HPLC
LCGC Europe
A simple rapid HPLC method for simultaneously determiing formononetin, biochanin A, daidzen and genistein in Red Clover
Trifolium pratense (red clover) is a medicinal herb, traditionally used in the treatment of chronic skin diseases and whooping cough, which contains at least four estrogenic isoflavones: formononetin, biochanin A, daidzen and genistein (Figure 1).1–3 Supplements containing isoflavones derived from red clover isoflavones are promoted worldwide for the treatment of menopausal symptoms and the maintenance of health and welfare after the menopause.4
In recent decades, red clover has been extensively investigated in phytochemistry, and the results indicated that formononetin, biochanin A, daidzen and genistein were the main active components. Pharmacological studies on the components showed that they all had good biological activities. Biochanin A has been reported to have beneficial health effects including antioxidant, anti-inflammatory and anticarcinogenic effects.5–7 Daidzen and genistein have a structure similar to the hormone estrogen and share some of its physiological properties. They may also act as endocrine disruptors.8,9
To our knowledge, previously reported analytical methods were developed to quantify only one or two types of components in red clover such as studying pharmacokinetics and bioavailability of the isoflavone biochanin A in rats with HPLC,7 determination of daidzen and genistein by GC–MS and HPLC,10,11 biochanin A and formononetin by HPLC–ELSD or diode array detector (DAD)12 and total biochanins A by HPLC–UV.13 In this study, a HPLC–DAD method was developed to quantify four components simultaneously in red clover. The parameters validated were linearity, quantification and detection limits, accuracy and precision.
Materials
Formononetin, biochanin A, daidzen and genistein were all of HPLC grade and purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Acetonitrile and methanol were of HPLC-grade and purchased from Fisher Scientific International (St Louis, Missouri, USA). Distilled and deionized water were used throughout the study. Red clover was collected in natural growth sites in Hubei, China. The samples were crushed and dried in an oven at 50 °C for 24 h before being subjected to extraction procedures.

Key Points
Chromatographic Conditions
Experiments were performed on a Shimadzu LC-10ATvp system (Shimadzu, Kyoto, Japan) consisting of a LC-10ATvp binary pump, a SPD-M10Avp diode array detector (DAD), a CTO-10Asvp column oven, a SCL-10Avp system controller and a Class-VP workstation. Data were collected and processed by Class-VP 6.10 software (Shimadzu). For chromatographic analysis, a Kromasil C18 column (4.6 mm × 250 mm, 5 µm) was used. The mobile phase was acetonitrile:water containing 0.5% phosphoric acid (40:60). The flow rate was 1.0 mL/min and column temperature was maintained at 30° C. The detection wavelength was set at 254 nm for acquiring chromatograms.
Standard Solutions
Based on the solubility of each component in methanol, stock standard solutions were prepared in methanol at concentrations of 0.25 mg/mL, 0.1 mg/mL, 0.75 mg/mL and 0.5 mg/mL for daidzen, genistein, formononetin and biochanin A, respectively. The working solutions for standard curves at specific concentrations were prepared by diluting the stock solutions with methanol:water (50:50) containing 0.5% phosphoric acid.
Sample Preparation
About 500 mg of the powdered sample was accurately weighed in a 50 mL volumetric flask followed by the addition of 20 mL of extraction solvent (methanol:water, 80:20 v/v, containing 0.5% phosphoric acid). The flask was shaken and placed at room temperature for 30 min, and then sonicated for 15 min. After cooling to room temperature, the mixture was made up to volume with the extraction solvent. An aliquot (1 mL) of the final mixture was further diluted with 1–10 mL of methanol:water (80:20) containing 0.5% phosphoric acid to appropriate concentrations, and then followed by centrifugation at 10000 g for 10 min. An aliquot (20 µL) of the supernatant was injected into the HPLC for analysis. The experiment was conducted in triplicate.
Chromatography
The development of a fast HPLC system with a sufficiently high efficiency is currently in demand in the field of chromatography. It has been well recognized that the column efficiency in the high flow rate ranges of the mobile phase solvent primarily rests on the mass transfer resistance in the stationary phase.14 When full-porous and non-porous fine spherical particles are used, there is a limitation to the mobile phase flow rate, suggesting that they are not suitable for fast HPLC. The pellicular spherical particles of a relatively large diameter and the full-porous cylindrical fibre have a separation speed advantage over the fine particles because they can make the size of macro-pore channels between the stationary phase materials wider. The reduction in the thickness of the superficial porous layer of the pellicular type particles and in the radius of the cylindrical fibre is effective for improving the column efficiency. Although the efficiency of the non-porous spherical particles is sufficiently high, they have two drawbacks; the extremely small retention strength and the narrow flow rate range of the mobile phase. These results suggest that the performance of the separation media should be comprehensively studied because the chromatographic behaviour is affected by their intrinsic characteristics concerning the retention strength, the mass transfer kinetics and the permeability against the mobile phase flow. In order to achieve simultaneous separation and determinate the four analytes, we chose a full porous column due to the column efficiency and peak capacity.12
A new and simple HPLC method was developed and validated for the quality control of red clover. The developed HPLC procedure is specific to four components in the red clover products. The chemical structures of the four components were shown in Figure 1. Under the current chromatographic conditions, the components were satisfactorily separated. Figure 2 shows representative HPLC profiles of red clover detected at 254 nm for four estrogenic isoflavones. No interference was observed under the assay conditions. The peak of each analyte was identified by comparing the retention time with that of standards and further confirmed by comparison of online UV spectra. Typical retention times for each component are listed in Table 1.
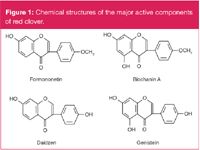
Figure 1: Chemical structures of the major active components of red clover.
Linearity, Limit of Detection (LOD) and Limit of Quantification (LOQ)
The linearity of the HPLC method was evaluated by formononetin, biochanin A, daidzen and genistein. Peak areas of the analyte were plotted. A calibration graph was constructed using the eight standard solutions. The weighted linear regression equations were listed in Table 1. As can be seen, the technique performed showed excellent correlation coefficients, and sensitivity.
The limit of detection (LOD) and limit of quantification (LOQ) for the four analytes' standards were determined based on the standards/baseline signal-to-noise (S/N) ratio. Four standard stock solutions were initially prepared. Dilutions and injections of these standards were then made until a HPLC chromatograph showed that the four peak heights reached an S/N of approximately 10:1 and 3:1 for LOQ and LOD solutions, respectively. Five injections of the LOQ and three injections of the LOD solutions were made and the %RSD for the LOQ solution was determined. The LOD and LOQ for the four analytes were determined shown in Table 1.

Table 1: Linearity and sensitivity for quantification of four components in red clover using the developed HPLCâDAD method.
Accuracy
Method accuracy was calculated by spiking five samples of red clover with standard stock solutions at the expected concentration of analyte. Two injections of each preparation were made and the theoretical amount of analyte in the sample preparations and the average percentage analyte recovered in the spiked solutions were calculated. The mean recovery for the four analytes was 96.20–102.75% with %RSDs less than 5%. Results are shown in Table 2.

Table 2: Extraction recoveries of the four studied components in red clover.
Precision
The precision injection was evaluated by repeated injection of the sample solution six times. The RSD values of peak area and retention time were better than 5%. The acceptable intra- and inter-day precisions (RSD) and accuracy (relative error, RD) were set as 10% and between 5% and 5%, respectively. Intra- and inter-day variabilities were determined by analysis of the average amount of standards in quality control samples prepared by standard solutions on three different days. The quality control samples were prepared as a single batch on the same day at each concentration, and then divided into aliquots that were stored at -20°C until analysis. The calculated relative standard deviation (RSD) and relative deviation (RD) values from repeated measurements were summarized in Table 3. The assay precision was ranged from 0.78–4.49%.
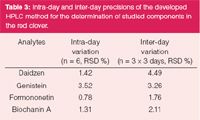
Table 3: Intra-day and inter-day precisions of the developed HPLC method for the determination of studied components in the red clover.
Intermediate Precision
Two sets of red clover samples (five of each) were prepared separately by two analysts. The samples were then analysed under the same experimental conditions with two separate columns and HPLC systems. The mean weight percentage and intra- and inter-day %RSD values for each analyte and between analysts and systems were calculated. The results were considered acceptable if the %RSDs between the different conditions were approximately 5%. Results showed that the %RSDs of these samples were at or within this limit for each analyte of interest.
Standard solution stability
Repeat injections of the mix of formononetin, biochanin A, daidzen and genistein standard solutions for 1, 2, 4, 6 and 8 h showed <2% RSD. Thus, the standard solution was determined to be stable at room temperature for over 8 h. Comparison of the response factors between a standard solution stored over 30 days in the refrigerator against a newly prepared one showed that the %RSDs were <5% for the four analytes. Therefore, the standard solution is considered to be stable for at least 30 days at the refrigerated storage conditions.
Applications
The quantification of the four analytes was performed using HPLC and the contents of four active components in red clover is shown in Table 4, which shows the different contents by HPLC analysis. This method has a shorter run time, simpler standard preparation and could be used for quantitative analysis and quality control of red clover and related products.
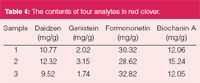
Table 4: The contents of four analytes in red clover.
Acknowledgements
These works were supported by the Foundation of the Education Department of Hubei Province (nos D20102108 and B20092410) and the National Scientific Foundation of Hubei (no. 2009CDB083).
Dr Qinhua Chen is pharmacist-in-charge specializing in pharmaceutical analysis and works in the Dongfeng Hospital, Hubei Medical University, Hubei, China.
Professor Peng Li, is associate chief pharmacist and specialises in pharmaceutical analysis and works in the Dongfeng Hospital, Hubei Medical University, Hubei, China.
Prof. Bing Li is a chief physician specializing in paediatrics and works in the Dongfeng Hospital, Hubei Medical University, Hubei, China.
Prof. Xiulou Li is associate chief physician specializing in medical statistics and works in the Dongfeng Hospital, Hubei Medical University, Hubei, China.
Jun Zhu, is pharmacist-in-charge specializing in hospital pharmacy and works in Dongfeng Hospital, Hubei Medical University, Hubei,China.
Fuchao Chen, is a pharmacist specializing in hospital pharmacy and works in Dongfeng Hospital, Hubei Medical University, Hubei, China.
References
1. J.T. Coon, M.H. Pittler and E. Ernst, Phytomedicine, 14(2), 153–159 (2007).
2. N.L. Booth et al., Menopause, 13(2), 251–264 (2006).
3. F. Occhiuto et al., Phytother Res.,21(2), 130–134 (2007).
4. F. Occhiuto et al., Phytomedicine, 15(9), 676–682 (2008).
5. Y.J. Moon et al., Pharm. Res.,25(9), 2158–2163 (2008).
6. Y.J. Moon, M.E. Morris, Mol. Pharm., 4(6), 865–872 (2007).
7. Y.J. Moon et al., AAPS J., 8(3), 433–442 (2006).
8. R.E. De et al., J. Chromatogr. A, 932(1), 55–64 (2001).
9. S. Moors et al., Mol. Nutr. Food Res., 51(7), 787–798 (2007).
10. S.L. Pumford et al., Ann. Clin. Biochem., 39(3), 281–292 (2002).
11. K. Mitani, S. Narimatsu, H. Kataoka, J. Chromatogr. A, 986(2), 169–177 (2003).
12. Q. Ma et al., Chin. Trad. Herb. Drug, 36(3), 372–375 (2005).
13. J.G. Zeng et al., Nat. Prod. Res. Dev., 23(1), 35–38 (2006).
14. K. Miyabe, J. Chromatogr. A, 1183(1–2), 49–64 (2008).
Characterizing Polyamides Using Reversed-Phase Liquid Chromatography
May 5th 2025Polyamides can be difficult to characterize, despite their use in various aspects of everyday life. Vrije Universiteit Amsterdam researchers hoped to address this using a reversed-phase liquid chromatography (RPLC)-based approach.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











