The QuEChERS Revolution
LCGC Europe
An interview with the inventors about the successes, challenges and potential future directions of this technique.
The technique of QuEChERS (quick, easy, cheap, effective, rugged and safe) is only seven years old, yet it is revolutionizing the manner in which multiresidue, multiclass pesticide analysis (and perhaps beyond) is performed. Columnist Ron Majors interviews inventors Michelangelo Anastassiades and Steve Lehotay about the successes, challenges and potential future directions of this growing sample preparation technique.
Sample preparation and analysis of trace compounds in difficult matrices has always been a challenge. In today's society, the drive to expand food production to feed an ever-increasing population has led to the increased use of a multitude of toxic pesticides in all countries of the world. Analysts have had to keep up with techniques to measure low levels of these pesticides, sometimes used indiscriminately for a wide variety of crops, including those for which they were not intended. Hence, methods for the trace analysis of multiresidues for multiclasses of pesticides have received a great deal of attention, especially in view of recent scares of contaminants showing up in our food supplies.
Sample preparation traditionally used liquid–solid extraction followed by liquid–liquid extraction and more recently, solid-phase extraction (SPE) to isolate pesticides of interest. In 2003, building on extraction techniques of the past, chemists working at the US Department of Agriculture introduced a technique that was simple, yet effectively isolated trace pesticides from a variety of fruits and vegetables.1 Relying heavily on marked improvements in high sensitivity, high selectivity tandem techniques of gas chromatography–mass spectrometry (GC–MS) and liquid chromatography–mass spectrometry (LC–MS) (and more recently on their MS–MS counterparts), the technique of QuEChERS (quick, easy, cheap, effective, rugged and safe, and pronounced 'catchers') was developed (Figure 1). An acetonitrile salting-out extraction of a solid sample in an aqueous environment is followed by liquid–solid extraction (called dispersive SPE, abbreviated d-SPE) to remove a majority of the remaining matrix interferences. The sample is then analysed by one or more of the tandem techniques.

Figure 1: Modern GCâMS and LCâMS instruments have been important in the development and growth of QuEChERS for sample preparation.
The two chemists who, along with their coworkers, developed and piloted the technique through the international validation and accreditation organizations [AOAC International and European Committee Standardization (CEN), respectively] were Doctors Steven J. Lehotay of the USDA in Philadelphia, Pennsylvania, USA and Michelangelo Anastassiades of Chemisches und Veterinaruntersuchungsamt, Stuttgart, Germany, who worked together in the USDA laboratory from 2000 to 2002. The technique has grown to the extent that it is becoming the standard sample preparation technique for pesticides in fruits and vegetables in laboratories across the globe. In addition, QuEChERS is rapidly expanding beyond its traditional applications area to address a wide variety of contaminants, antibiotics, pharmaceuticals, drugs of abuse and other compounds in a wider variety of matrices such as meat, fish, whole blood, wine and even soil. For this instalment of "Sample Preparation Perspectives", LCGC Europe columnist Ron Majors interviewed Anastassiades and Lehotay about the technique, its successes and challenges and where it might be heading in the future.
Major Improvements
The original QuEChERS publication was in 2003. What would you both consider to be the three biggest improvements in these extraction protocols since the original method was developed?
During the conception of QuEChERS, we conducted many experiments to isolate each facet of the extraction and clean-up process for a wide range of representative pesticides and commodities. The original method from the 2003 publication1 is fine and even advantageous over current modifications, depending upon the analytes and matrices. When we independently conducted validation experiments for hundreds of pesticides in different commodities, we both found that there were about 10 pesticides that showed pH dependence in the recoveries, depending on the matrix.2–4 Buffering at pH = 5 was the best compromise to achieve acceptable recoveries for those few pH-dependent pesticides and we each introduced buffering during extraction in our preferred way.3–7 This was the first 'improvement' (however, buffering also leads to more coextractives in some matrices, so we prefer to call it an 'adjustment' rather than an 'improvement').
Secondly, the addition of C18 along with the primary-secondary amine (PSA) in the d-SPE step definitely helps improve clean-up for some samples, particularly those that contain lipids such as olives,8 and it does no harm in any case. Lehotay regrets that he did not uniformly include C18 in the AOAC Official Method 2007.01 protocol, while in the European Standard Method EN 15662, C18 was made an option. Some chemists employ a freeze-out step to reduce lipid coextractives, but C18 in d-SPE is faster and easier, and has been shown to work equally well in removing lipids, although freezing out also precipitates additional matrix components having limited solubility in QuEChERS extracts.4,9,10
Thirdly, the reduction of chlorophyll coextractive from green commodities and carotenoids via the addition of a small amount of graphitized carbon black (GCB) as in the CEN Standard Method 156627 or ChloroFiltr product (UCT Inc., Bristol, Pennsylvania, USA) constitutes a third prominent adjustment, at the sacrifice of ≈25% reduced recovery of some structurally planar pesticides. Another option of note entails the use of a greater amount of PSA in d-SPE to remove more fatty acid coextractives from cereals and grains (at the expense of ≈20% lower recovery of certain polar pesticides).11
Major Difficulties
What have been the three biggest setbacks or difficulties in advancing the QuEChERS methodology?
We don't think we've really had any setbacks in advancing QuEChERS concepts and the number of vendors selling QuEChERS and d-SPE products has continued to increase (Table 1). QuEChERS generated much excitement from the day it was introduced at the 4th European Pesticide Residue Workshop (2002 in Rome) and it has continued to gain popularity since (Figure 2). For example, Anastassiades has compared QuEChERS with the traditional method extensively used throughout Europe for 15–20 years12 and calculated an approximate 95% reduction in solvent consumption (10 versus 535 mL), an approximate 95% reduction in consumable costs, and an approximate 90% reduction in time. Looking at the reports of the official EU-proficiency tests for pesticides in fruits and vegetables with 130–150 participants each year, the number of laboratories using QuEChERS has been continually increasing to reach approximately 40% in 2009. Among private laboratories, where financial aspects weigh even more, the adaptation rate to QuEChERS is reportedly even greater and close to 70%.

Table 1: QuEChERS vendors and web addresses.
The biggest difficulty with QuEChERS arises from its greatest strength: it is extremely flexible and rugged in the sense that dozens of different small modifications will still give high recoveries for hundreds of pesticides in hundreds of commodities. There is an old expression that "analytical methods are like toothbrushes and everybody prefers to use their own." We can see numerous modifications of the original method or the two official versions of QuEChERS in the literature that are based solely upon personal preferences or limited experimentation. Sometimes modifications are necessary to better adjust the method to a specific application, but in many cases the impression is that the introduction of amendments arises merely from the need to do something different to better justify projects or publications. In various cases, modifications actually make the method less rugged, longer, or more expensive to perform.
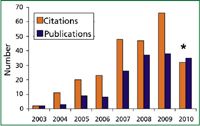
Figure 2: Number of citations to reference 1 by year and publications with topic "QuEChERS" or dispersive solid-phase extraction, according to ISI Web of Knowledge search conducted on June 3 2010. The asterisk indicates partial results for 2010. Total = 249 citations and 158 publications.
We went through this divide ourselves when Anastassiades preferred to use citrate buffering (at a relatively low buffering capacity)7 while Lehotay preferred acetate buffering (at higher concentration to give greater buffering strength).3,6 We each have our reasons, but ultimately both methods have been extensively validated for many pesticides in many foods by many laboratories.
Another difficulty inherent in the flexibility of the method is that it accommodates different sample sizes and different injection volumes and solvents. Therefore, the vendors provide many products to accommodate the many preferences and options that laboratories use throughout the world. Too many details and too many choices also lead to misinformation and confusion among novices in the pesticide analysis field.
Perhaps the greatest difficulty that all multiclass, multiresidue methods possess is that certain pesticides (for example, captan, captafol, folpet and chlorothalonil) don't give high and consistent recoveries with the method. Acidic conditions help to reduce losses of these pesticides, but these analytes degrade readily in nonacidic samples even before extraction. Unfortunately, these troublemaker compounds are not amenable to LC–MS–MS and their GC analysis is notoriously problematic. The GC–MS conditions have to be well controlled for their high-quality detection, which is not always possible in routine monitoring operations. Therefore, QuEChERS faces criticism by some for not being perfect. The use of analyte protectants (APs), another interesting feature introduced in the original QuEChERS paper, improves GC analysis of these troublemakers.1,13,14
Harmonization
You both worked on the original methods, yet as you have gone your separate ways, your refinements have led to two additional and different extraction protocols. Currently, there are three popular "standardized" methods: original, AOAC Official Method 2007.01 (attributed to Lehotay and coworkers) and CEN Standard Method EN 15662 (attributed to Anastassiades and coworkers, see Figure 3), along with many homemade modifications. Is there a movement to 'harmonize' these methods to provide the users (and the manufacturers who supply kits) a more generic choice?
According to Lehotay, AOAC International has approved moving forward with another collaborative study to 'harmonize' the QuEChERS method in which the best of both current official methods are combined into a single method for a variety of foods. This entails the use of acetate buffering during extraction and d-SPE (or disposable pipette extraction, DPX, DPX Laboratories, Columbia, South Carolina, USA) clean-up with 50 mg PSA, 50 mg C18 and 7.5 mg GCB (plus 150 mg MgSO4 drying agent) per millilitre of extract. For cereals and grains, a 2.5 or 5 g sample (plus 10 mL water) is extracted and 150 mg of PSA is used rather than 50 mg. The study protocol still needs to be written and reviewed by the AOAC Int. Chemical Contaminants and Residues in Food Community.

Figure 3: Steps in the original and official versions of QuEChERS sample preparation of pesticide residue analysis of food commodities.
Expanding Analytes/Matrices
Although QuEChERS started out as a method for the extraction of multiclass, multiresidue pesticides from fruits and vegetables, it now is being used for a wide variety of analytes (for example, vet drugs, PAHs and antibiotics) in a wide variety of matrices (for example, plasma, meat and soil). Do you think that it is expanding too quickly without the careful interlaboratory testing that you performed during your early work?
We are glad to see the exploration and expansion of QuEChERS concepts in different applications. In terms of quality of the work, it is a case-by-case basis, which boils down to good science or not, independent of QuEChERS. In all endeavours, people have to critically assess the quality and validity of the information they are given.
From the beginning, we did not try to patent or control the use of QuEChERS concepts, foremost because we did not think the chemical approach or d-SPE could be patented (after all, Tswett used sorbents in tubes for chromatography prior to the idea to use a column), but also because philosophically, we both feel that QuEChERS would be more widely used if we (or our respective employers) did not become involved in business. Neither of us personally nor our organizations profit monetarily from the QuEChERS name or selling of commercial products.
What we have done to "protect" QuEChERS is work through the auspices of international official method validation and accreditation organizations (AOAC International and CEN). AOAC Official Method 2007.01 and CEN Standard Method EN 15622 are gold standard methods and modified versions are acceptable if they are properly validated and fit-for-purpose in the particular application.
However, we caution that many methods achieve high and consistent recoveries for spiked samples during method development and limited validation experiments, yet the method may not work well in real-world samples in which the analyte–matrix interactions are stronger or routine analysis poses instrument maintenance problems. For example, we are sceptical of methods using QuEChERS for soil analysis because stronger extraction conditions are typically needed, such as pressurized liquid extraction (PLE) or Soxhlet extraction, rather than just shaking to overcome the strong binding characteristics of the soil–sediments. Pesticide extraction from many foods does not entail strong matrix interactions, and shaking has been demonstrated to be acceptable for incurred as well as spiked samples.
Expanding Applications
Along those lines, the original QuEChERS was devoted to trace pesticides, mainly in pesticide laboratories that were very familiar with the techniques of statistical and representative sampling. As QuEChERS finds its way into nonpesticide laboratories that are doing trace analyses but without the wisdom that pesticide analysts have in that area, do you think that QuEChERS might get a "bad rap" because of nonrepresentative samples being analysed? After all, the result is only as good as the sample supplied.
Again, representative sampling issues are fundamental considerations with any analysis, not just with QuEChERS. In our original paper,1 subsequent papers and all presentations we've given about QuEChERS, we emphasize that the original sample collection and homogenization step is integral to the QuEChERS concept due to the smaller subsample size and use of shaking for extraction. In this regard, the $5000 chopper used for sample comminution is more important than the $300000+ worth of LC–MS and GC–MS instruments used for analyses.
Automation
With pesticide laboratories now encountering large numbers of samples, the question of automation of the entire QuEChERS process naturally arises. In its generally practiced form, QuEChERS is still a manual procedure with lots of shaking and sample manipulations. How do you see the automation of the technique taking hold?
In our views and experiences, QuEChERS is so fast and easy that, rather than making it quicker and simpler, automation adds to the time and effort for sample preparation, and certainly adds to the expense. A chemist can often complete the procedure by hand for a batch of samples faster than a person can load and unload the tubes in automated systems. Fortunately, we have large enough hands to hold 6–10 extraction tubes (50 mL) at a time, and enjoy the 1 minute exercise from the vigorous shaking. However, one of the most frequent complaints from bench chemists is that they don't like the shaking step (despite the suggestion for them to put on some music and do the QuEChERS dance!). Also, some people just don't shake as vigorously as others.
Therefore, a strong shaker can be a beneficial tool in the laboratory for the extraction step, depending on analyst's capabilities, and commercial shakers are an option for QuEChERS. Anastassiades is currently studying the impact of extraction conditions on the extraction yields of incurred pesticide residues from food commodities. In many cases, 1 minute extractions are just fine, but there are also cases where there is a notable increase in the extracted amounts when extraction time is extended. For example, this effect may concern residues that have diffused into wax layers. Wax particles obviously need some time to soak for those enclosed residues to become accessible to the extractant. Automated shakers thus become very interesting for QuEChERS applications from this point of view as well.
Otherwise, the d-SPE step does not require vigorous shaking and it only takes 30 s, thus it does not need automation. The semi-automated DPX device can handle 20 samples at a time, which does not slow sample throughput versus manual d-SPE. The use of automated robotics to conduct d-SPE may save time in theory, but the logistics, costs and added instrument downtime seem daunting for little benefit.
The most laborious and time-consuming sample processing and sample weighing steps seem beyond the ability to automate and the little details of entering information about samples and keeping track of them are also of critical importance and require careful human inputs.
Now that QuEChERS is commonly used for sample preparation, the limiting step for sample throughput involves data analysis — reviewing the results for hundreds of pesticides in the analyses. The data processing software is still not trustworthy enough in our opinion to allow it to generate results without human review. Residue chemical analysis does not currently entail the same degree of high-throughput sample analyses that clinical or pharma tests have employed, but if QuEChERS is used in those type of applications, then its automation becomes more important. Gerstel (Germany, UK and US) is working with DPX Labs and others to automate QuEChERS.
Matrix Dependencies
Extractions of all kinds are often matrix dependent. An analyte in one matrix can behave well but in another matrix be difficult to extract. Have you seen cases like this in QuEChERS? If so, are there some recommended modifications in the QuEChERS protocols to handle these situations?
Food samples in most cases do not have pesticide–matrix interactions to affect recovery (certain pesticides in dry and oily samples are some exceptions). However, there are some complex matrices (such as teas, herbs, spices, liver, citrus oil and the like) that give a tremendous number of coextractives no matter what method is used. It is inherent in multiclass, multiresidue methods that the sample preparation process cannot be so selective to remove many matrix chemical compounds if those chemicals have similar properties as the diverse range of analytes being monitored. Sure, modifications can be made by adjusting solvents, pH, salts, volumes, water content and clean-up sorbents.
In terms of pH adjustment, for example, Anastassiades found out that the quantitative extraction of nicotine from mushrooms requires pH values in the range of 10–11.15–16 However, the extraction of chlorothalonil from certain commodities, such as onions and leeks, requires pH values close to 2 to minimize losses caused due to interaction with the matrix. Similarly low pH values are needed to achieve good recoveries for pesticides belonging to the nereistoxins class.
Acidic herbicides, such the phenoxyalcanoic acids, tend to form covalently bound residues. As these are typically included in legal residue definitions, they have to be released before extraction. This is conveniently achieved by introducing an alkaline hydrolysis step (30 min at pH 12) before reneutralization and normal QuEChERS extraction.15,17
Individual pesticide classes can be better isolated through more intensive clean-up, but this inevitably reduces recoveries of other analyte classes and typically leads to fractionations into multiple extracts and multiple injections.
For such complex commodities, we thus very much rely on high quality chromatography–MS analysis using highly selective and sensitive modern instruments. High sensitivity instruments allow us to reduce the 'sample concentration' in the final extract (for example, to 0.1 g/mL in the case of spices) and thus also the sample equivalents injected, whereas the high chromatographic and MS selectivity allows us to largely 'fade-out' matrix components coeluting with pesticides (for example, terpenes in GC applications). If ruggedness or detection limits are compromised too much, then traditional methods involving gel permeation chromatography (GPC) or fractionation of extracts might be a better option. Such decisions depend on the required data quality objectives that need to be met and the practical matters of time, cost, labour and instrumentation in the laboratory.
We have never claimed that QuEChERS works well for everything, and we only say that it is fast and easy to try. If it is found to work well in the application, then it saves a tremendous amount of method development time and labour.
Detection Possibilities
QuEChERS extracts can be relatively 'dirty'. Having selective, sensitive detection such as MS and MS–MS helps provide the analytical selectivity on the back end of the analysis. For labs that can't yet afford these rather expensive detectors and are stuck with flame ionization detection (FID) in GC and UV–diode-array detection (DAD) in LC, do you recommend that they even try to use QuEChERS?
Even historically, FID and UV–DAD have not been selective enough for use in pesticide residue analysis. Instead, selective detection methods such as flame photometric detection (FPD), pulsed flame photometric detection (PFPD), nitrogen–phosphorus detection (NPD), electrolytic conductivity detection (ELCD), electron-capture detection (ECD), halogen-specific detection (XSD), and so forth have been used in GC, and postcolumn derivatization fluorescence in LC. Before LC–MS–MS, a wide range of polar pesticides was largely ignored in monitoring programmes because the single-analyte methods were too cumbersome in practice. The previous multiclass, multiresidue methods were designed for GC analysis, and QuEChERS combined with LC–MS–MS helped advance pesticide residue monitoring capabilities.
With LC–MS–MS or GC–MS–MS (or selective ion monitoring [SIM]) analysis, we rarely even notice the coextractives or observe interferences in typical commodities (except with spices and citrus perhaps). Similarly, QuEChERS with selective detectors in GC and LC does not provide much difference in terms of interferences than with prior methods. As described earlier, QuEChERS can be adapted by adjusting extraction and clean-up parameters for a narrower range of analytes.
GC–MS and especially LC–MS–MS are nowadays considered as indispensable for the effective control of pesticide residues. A large number of pesticides giving rise to toxicology concerns and trade restrictions are essentially only amenable to LC–MS type applications. We think that bodies offering technical and financial assistance to developing countries for the establishment of export-control laboratories are well-advised to help these labs to directly embark into LC–MS–MS rather than to scroll through the entire history of instrumental analysis by starting with basic techniques such as LC–DAD, GC–NPD and GC–ECD. The capital investment might be larger but in the long run LC–MS–MS is clearly worth the money by covering a broader scope of pesticides including most critical ones, thus improving the recognition of export certificates and reducing problems with consignment-rejections.
Now for some specific questions about the procedures:
During the initial acetonitrile extraction, a good bit of heat is generated by exothermic hydration during the addition of anhydrous MgSO4 to the samples. In one sense, this heat generation aids the extraction speed or efficiency while, on the other hand, too much heat may lead to a loss of volatile or thermally labile pesticides. In practice, how to you reconcile these conflicting occurrences? Or, isn't it an issue?
The recoveries have been shown to be fine for hundreds of pesticides and other analytes in the method. The heat causes potential degradation losses of the same few pesticides mentioned earlier, but acidification helps to stabilize these pesticides. When using frozen samples (as advised), the temperatures reached after extraction are very moderate.
Pesticides
I've heard that at last count there are over 400 pesticides in various matrices that have been extracted using QuEChERS. Besides the planar ring pesticides that are strongly retained by graphitized carbon black during the d-SPE step, are there any other classes of pesticides that cannot be extracted (or at least have very poor recoveries) using these official QuEChERS methods? If so, what alternatives do the users have for extracting these troublesome ones?
Anastassiades and coworkers have developed a database that allows the systematic collection and on-line retrieval of recovery data generated within the frame of coordinated and individual validation studies.18 This database currently contains more than 150000 individual QuEChERS recovery figures concerning more than 650 different pesticides and metabolites that were spiked to more than 115 different food commodities. This database, which also contains recovery data obtained by other common multiresidue methods, is a good source of information for analysts that would like to quickly check the performance of different methods in the analysis of various pesticide–commodity combinations. Of course, there are many pesticides that cannot be extracted through the full QuEChERS method, and those are the same ones that are traditionally analysed using single-analyte or single-class methods (for example, glyphosate and its metabolite AMPA, chlormequat/mepiquat/paraquat/diquat, fosetyl, ethephon, maleic hydrazide). Anastassiades is working to devise conditions to analyse these classes of pesticides into a single method.15
SPE Cartridges
Although d-SPE is the normal approach in QuEChERS, I've noticed that some workers, particularly in Asia, still prefer to use SPE-like cartridges such as the double-layered devices (for example, GCB/PSA) to accomplish this stage of clean-up, sort of a 'chemical filtration'. Why do some prefer this approach? Do you see the use of SPE cartridges, pipettes, discs and so forth increasing compared to d-SPE?
Yes, column SPE tends to give greater removal of coextractives than d-SPE, with an associated reduction in recovery of some polar analytes and more cost and time involved. We considered traditional SPE cartridges using chemical filtration even before QuEChERS, but the MgSO4 tended to clog the frits. It is important to use the drying agent in the d-SPE clean-up step to reduce water content from ≈7% to ≈2% in the final extracts (this helps in clean-up with PSA and reduces the amount of water being introduced into the GC system). Much lower residual water contents and thus even better clean-up effects result when using calcium dichloride instead of magnesium sulphate. This approach has been successfully used for the clean-up of fermented-tea extracts at the cost, however, of some 20% recovery losses of the most polar pesticides such as methamidophos.4 We also looked into 'filtering' the extracts through SPE columns or discs with a syringe, but we prefer d-SPE. DPX is another option, which provides filtering (2 µm) the final extracts.
It has always been an option to use traditional SPE for clean-up in QuEChERS, but we found that d-SPE gives higher recoveries of a wider scope of pesticides faster, easier and cheaper. Many take SPE for granted, but when one thinks about it, traditional SPE uses a manifold, a vacuum source, pre-treatment steps (conditioning, loading, washing, elution), collection tubes, flow control, solvent evaporation, careful attention and, at times, laborious method development by the analyst.
Dispersive SPE Phases
Do you foresee the need for additional dispersive SPE phases or will the large number of currently available SPE phases fill the need? If you could design the 'perfect' adsorbent for dispersive SPE what would it have to do?
There are as many sorbents for use in d-SPE as there are in cartridge-SPE. The perfect sorbent removes only matrix components in the final extract solvent and does not retain the analytes. In all foods, these matrix components consist of lipids, carbohydrates, proteins, water and lesser amounts of minerals, vitamins and a variety of natural products, depending on the food. The selective extraction process of QuEChERS reduces the bulk coextractives (lipids, water, protein, sugars) and the d-SPE step is designed to further reduce the remaining constituents of fatty and other acids, chlorophyll, anthocyanidins and other pigments, sterols, water and sugars that may interfere in the analysis, cause matrix effects, lower recoveries and reduce instrument ruggedness.
The combination in d-SPE of 150 mg MgSO4, 50–150 mg PSA, 50 mg C18 and 7.5 mg GCB per millilitre of extract is the most effective combination we know for pesticides in foods that still gives high recoveries with wide scope. Other sorbents and amounts, or pH and solvent adjustments or hexane partitioning, may remove certain matrix components more completely, but this tends to sacrifice recoveries of some pesticides. Molecular imprinted polymers (MIPs) designed to remove specific matrix components would be a great benefit if they also do not reduce analyte recoveries.
Disadvantages
There is a lot of talk about the advantages of QuEChERS — what are some of the disadvantages of this extraction technique?
The disadvantages (and advantages) of QuEChERS depend on what it is being compared to. We think QuEChERS has been found to be so advantageous over other options by so many laboratories that its disadvantages involve personal preferences and nuances, as discussed above, more so than inherent drawbacks. Without doubt, QuEChERS has changed the way in which sample preparation is conducted in pesticide residue analysis and even laboratories that aren't using QuEChERS per se, are using single tube extractions or d-SPE as part of their methods.
There is one issue of note, however, before QuEChERS, final extracts in pesticide residue methods were typically 2–5 g/mL sample equivalents in a nonpolar solvent, which led to 10 ng/g detection limits when using 1–3 µL splitless injections in GC–MS (in SIM mode). Unless the chemist uses the longer option to take a larger aliquot of the initial extract followed by a concentration and solvent exchange step, the QuEChERS method yields final extracts of 1 g/mL in acetonitrile. To achieve the same detection limits as before, programmable temperature vapourization (PTV) is needed for large-volume injection (LVI) of 3–10 µL. Many laboratories in North America don't use PTV-LVI in GC, but it has been a standard injection method in Europe for many years. Agilent's recent introduction of their multimode inlet may change this situation in the US, simply due to Agilent's sway in the market, because equivalent types of injectors have been commercially available for many years. It has always been a disappointment to Lehotay that Americans did not keep up with the Europeans in this regard.
Also, acetonitrile is an excellent solvent for LC methods, but it is not ideal for GC. The use of PTV-LVI reduces the amount of solvent passing through the GC column, so again, these aren't disadvantages if appropriate adaptations are made, but acidified acetonitrile has been observed to cause greater column bleed. Some base-sensitive pesticides degrade in acetonitrile (or at least in some solvent lots), thus we acidify the acetonitrile to increase stability of those types of pesticides. Acetonitrile is more expensive than other solvents, and it went through a shortage last year, but the QuEChERS method only uses 10–15 mL per sample.
The coextraction of chlorophyll is a potential disadvantage, and the 7.5 mg GCB or 50 mg ChloroFiltr per millilitre of extract only removes 80–90% of it. GPC may provide a better removal of certain large-sized matrix components compared to d-SPE, but GPC has worse disadvantages in terms of time, cost, and extensive solvent usage. Even in terms of fat removal d-SPE with C18 or freeze-out were shown to perform even slightly better than GPC.4,10
The worst problem in just about any method using LC–atmospheric pressure ionization (API)-MS for complex samples relates to ionization effects due to coeluting matrix components. Most foods do not give this problem for QuEChERS extracts and we are not aware that QuEChERS poses worse problems than other methods for highly complex matrices. Modern instruments have enhanced sensitivity and better ion source designs to help reduce this problem, but ionization charge competition is an inherent aspect of API. Isotopically labelled internal standards cannot be used for all analytes, thus chemists have to assess matrix effects and be wary of uncorroborated quantitative LC–API-MS results until a solution is found.
Future
About the future, where do you see QuEChERS going? What challenges lie ahead?
The FDA Luke method19 and German Specht method12 lasted about 20–25 years as the most common approach for pesticide residue analysis, and at today's faster pace of technological development, we'd be surprised if QuEChERS lasts as long. We would like to see QuEChERS concepts continue to be tested and validated in new applications, but we don't know what those will be. We expect that d-SPE will be useful in environmental applications, but question the use of shaking for soil extractions. Veterinary drug residues, clinical, and forensic applications seem like a good fit for QuEChERS as multiclass, multiresidue analysis becomes more accepted due to LC–MS technologies.
Recently, Lehotay was pleased to see that Graham Cooks and his students at Purdue University were using QuEChERS as the sample preparation procedure for their novel ambient MS approaches that bypass chromatography.20 QuEChERS helped to reduce background matrix levels and to provide an extract that yielded a meaningful result.
QuEChERS blossomed due to an increased usage of modern chromatography and MS instruments, and sample preparation will continue to evolve in conjunction with analytical detection techniques. As we already discussed, collecting and processing a representative sample is just as important as the detection, and as analytical chemistry continues to push into the mini, micro and nano realms, the sample preparation approach giving meaningful results will become even more difficult. If one day hundreds of data points from diverse parts of a representative sample can be obtained very quickly in the field, we suppose that would be one way around sample preparation, but for multiclass, multiresidue analysis, this seems a long way into the future.
Ronald E. Majors is "Sample Prep Perspectives" Editor and Senior Scientist, Columns and Supplies Division, Agilent Technologies, Wilmington, Delaware, and is a member of LCGC Europe's editorial advisory board. Direct correspondence about this column should go to Alasdair Matheson, LCGC Europe, Advanstar Comunications, Poplar House, Park West, Sealand Road, Chester CH1 4RN, UK. e-mail: amatheson@advanstar.com.
Steve Lehotay has BS and PhD degrees in chemistry from the University of Florida and has worked in the USDA Agricultural Research Service since 1992. His main research focus involves the improvement of methods used in the analysis of pesticides, veterinary drugs, and other contaminants in foods. He has authored or co-authored more than 100 scientific publications and 150 abstracts.
Michelangelo Anastassiades studied food chemistry at the University of Stuttgart, Germany and earned the PhD degree from the University of Hohenheim, Germany. He has been working in the field of pesticide residue analysis since 1995. From 2000 to 2002, he worked at the USDA as a visiting scientist. Since 2006, he has been acting as head of the EU Reference Laboratory for pesticide residue analysis using single residue methods, which is hosted at the CVUA Stuttgart.
References
1. M. Anastassiades et al., J. AOAC Int., 86, 412–431 (2003).
2. S.J. Lehotay et al., J. AOAC Int., 88(2), 595–614 (2005).
3. S.J. Lehotay, K. Mastovska and A.R. Lightfield, J. AOAC Int., 88(2), 615–629 (2005).
4. M. Anastassiades et al., Pesticide Chemistry: Crop Protection, Public Health, Environmental Safety, H. Ohkawa and L. Miyagawa, Eds. (WILEY-VCH Verlag GmbH Co. KGaA, Weinheim, 2007), pp. 439–458.
5. P. Payá et al., Anal. Bioanal. Chem., 1697–1714 (2007).
6. S.J. Lehotay, J. AOAC Int., 90, 485–520 (2007).
7. EN 15662: Determination of Pesticide Residues Using GC-MS and/or LC-MS (/MS) following Acetonitrile Extraction/Partitioning and Clean-up by Dispersive SPE - QuEChERS method. In European Committee for Standardization, Technical Committee CEN/TC 275; "Food analysis - Horizontal Methods"; Brussels, Belgium, 2008.
8. S.C. Cunha et al., J. Sep. Sci., 30, 620–632 (2007).
9. S. Walorczyk, J. Chromatogr. A, 1208, 202–214 (2008).
10. U. Koesukwiwat et al., J. Agric. Food Chem., 58, 5950–5958 (2010).
11. K. Mastovska et al., J. Agric. Food Chem., 58, 5959–5972 (2010).
12. W. Specht and M. Tilkes, Fresenius. Z. Anal. Chem., 322, 443–455 (1985).
13. M. Anastassiades, K. Maštovska and S.J. Lehotay, J. Chromatogr. A, 1015(1–2), 163–184 (2003).
14. K. Maštovska, S.J. Lehotay and M. Anastassiades, Anal. Chem., 77, 8129–8137 (2005).
15. www.crl-pesticides.eu/docs/public/tmplt_article.asp?LabID=200&CntID=670&Lang=EN
16. www.crl-pesticides.eu/library/docs/srm/meth_NicotineMushrooms_CrlFvCrlSrm.pdf
17. www.crl-pesticides.eu/library/docs/srm/acidicpesticides_wheat_quechers.pdf
18. M. Anastassiades et al., Method Validation Database by EURL Single Residue Methods. In http://www.crl-pesticides-datapool.eu: 2002-2010.
19. L.D. Sawyer, J. Assoc. Off. Anal. Chem., 68, 64–71 (1985).
20. J.S. Wiley et al., Analyst, 135, 971–979 (2010).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)






