Separation of Steviol Glycosides
Knauer Application Note
A robust, selective and sensitive separation method for the determination of four steviol glycosides in stevia plant matrix is demonstrated. In addition to the two calibrated substances two more glycosides could be found in stevia plant extracts by LC–MS. Using a popular Eurospher NH2 phase and applying moderate separation conditions results in an inexpensive and precise analysis method.
Introduction
Steviol glycosides are responsible for the sweet taste of stevia plant leaves. These compounds are 75 to 450 times sweeter than sucrose without the typical bitter aftertaste of some well-known artificial sweeteners. In the course of the accreditation of these four steviol glycosides by the public health authority a stable and sensitive analytical quantitative separation method is highly recommendable.
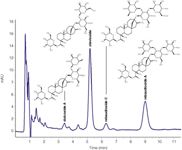
Figure 1
Experimental
The separation was performed in isocratic mode on a KNAUER Smartline HPLC system with low pressure gradient capability, degasser, autosampler and column oven.
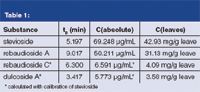
Table 1
Results
The separation could be achieved in 10 minutes. The limit of detection (LOD, S/N = 3) was determined for stevioside at 7.5 ng and for rebaudioside A at 16.0 ng.

Table 2: Method performance.
Conclusion
A fast and very good separation of four steviol glycosides in Stevia leaf extract samples with excellent peak symmetry is easily accomplished by reversed-phase HPLC using the Eurospher NH2 column in combination with a Smartline HPLC system. The simple isocratic method with moderate parameters guarantees robust and sensitive results over a long time period. The method allows safe quantification. Contact info@knauer.net for a more detailed version of this application.
Knauer GmbH
Hegauer Weg 38, 14163 Berlin, Germany
tel. +49 30 809727 0 fax +49 30 801501 0
E-mail: info@knauer.net
Website: www.knauer.net

A Matrix-Matched Semiquantification Method for PFAS in AFFF-Contaminated Soil
Published: April 14th 2025 | Updated: April 14th 2025Catharina Capitain and Melanie Schüßler from the Faculty of Geosciences at the University of Tübingen, Tübingen, Germany describe a novel approach using matrix-matched semiquantification to investigate per- and polyfluoroalkyl substances (PFAS) in contaminated soil.
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.











