Separation Science in Drug Development, Part I: High-Throughput Purification
LCGC North America
This installment provides an overview of modern practices of high-throughput purification to support small-molecule drug discovery.
This installment provides an overview of modern practices of high-throughput purification to support small-molecule drug discovery. It describes the use of reversed-phase liquid chromatography (LC) and supercritical fluid chromatography (SFC) for purifying diverse samples in a centralized laboratory setting. A case study is used to illustrate the principles and rationales for selecting operating parameters for these applications. This is the first installment of four articles on "Separation Science in Drug Development" to describe the modern practice of separation science in supporting small-molecule drug discovery and development. The complete series will be published throughout the year in LCGC North America.
Drug development is a highly complex, expensive, and multidisciplinary process (1,2). The modern molecular approach to new drug development generally starts with an understanding of the pathophysiology of a disease and a molecular target, followed by the synthesis of molecules that bind to the target (hits and leads), which are subsequently optimized by medicinal chemists (3) into a development candidate. The drug candidate is then scaled up by process chemists, characterized by analytical chemists, and formulated by pharmaceutical scientists. If safety and efficacy profiles of the drug candidate are acceptable, it may eventually become a new commercial drug product after a stringent regulatory approval process. Analytical chemistry, particularly the separation science, plays an important role throughout this long and arduous process (4,5).
Because the lifetimes of patents for new drug molecules are relatively short (for example, 20 years in the United States), the speed in selecting the "right" candidates and the subsequent reduction in "time to market" are important factors that can impact the survival of the pharmaceutical company. In early discovery, it is vital to minimize the time between compound design and assay results, which are subsequently used to drive the next round of compound design in an iterative process called the "drug discovery cycle" (shown in Figure 1).
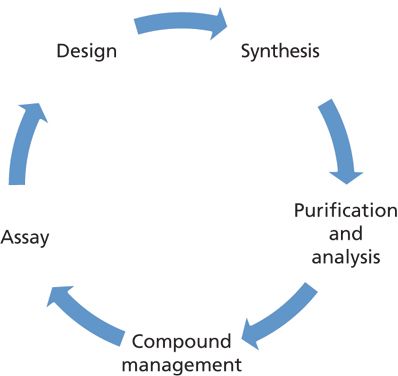
Figure 1: The drug discovery cycle.
In the drug discovery cycle, medicinal and computational chemists are typically involved in hypothesis generation and compound design. Medicinal chemists are responsible for the synthesis of test compounds; analytical chemists for their purification and analysis (characterization); and compound management for weighing, creating stock solutions, and storing the compounds for future testing. Assays that are performed by multiple groups include physicochemical property screening, biological testing, and drug metabolism and pharmacokinetics. Assay results are used to generate the next hypothesis–compound design cycle in an iterative fashion.
In this installment, we describe the modern practice and recent advances in the high-throughput purification of compounds of interest (COI) in this drug discovery cycle. The goal of a high-throughput purification laboratory is to provide purified materials in sufficient quantity and quality (chemical and chiral purity) to support candidate characterization and further biochemical assays. The next installment will focus on the high-throughput characterization process to generate quality control and analytical data for internal submissions to "compound management."
Additional details of the typical processes and Genentech's sample workflow in a small-molecule discovery chemistry organization are summarized in Figure 2. The analytical chemistry functions of purification and analysis (shown in blue boxes) are often handled by a centralized analytical group, although many of these tasks can be done with either open-access instrumentation or can be outsourced to external contract research organizations (CROs). A compound management group is often responsible for archiving the entire portfolio of compounds and distributing them for testing, typically as stock solutions (for example, 10 mM in dimethylsulfoxide [DMSO]).
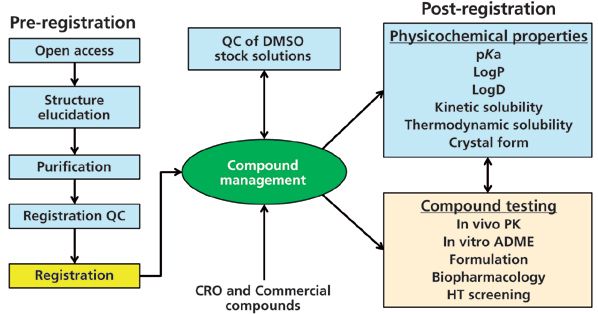
Figure 2: Sample workflow and processes in a small molecule discovery chemistry organization. The light blue boxes represent the functions likely to be performed by a centralized analytical chemistry group.
A challenge in a centralized analytical laboratory such as ours is the diversity of compounds received for purification. Although most samples are drug leads or active pharmaceutical ingredients, they can also be synthetic starting materials, intermediates, small polar fragments (<150 molecular weight [MW]), or linkers used with antibody–drug conjugates (>1500 MW). Another challenge is that many compounds have one or more chiral centers, which complicate the purification strategy as high chiral purities are prerequisites for ensuring the accuracy of assay results. Samples are submitted to the purification laboratory as crude mixtures in vials, flasks, or multiwell plates from parallel chemistry efforts. The goal is to generate a pure powder of sufficient quality (typically >90% pure with no single impurity >5%) for compound management. Having an analytical chemistry group in close proximity to medicinal chemists is an important benefit for shortening the drug discovery cycle through quick communication and sample transfers.
High-Throughput Purification Strategy and Workflow
Figure 3 shows a schematic diagram of the purification workflow used in our laboratory that may exemplify processes used in other centralized groups (6). Four major steps are used: initial screening; purification by reversed-phase liquid chromatography (LC) or supercritical fluid chromatography (SFC); quality control (QC) of collected fractions; and sample recovery. Achiral compounds are separated by reversed-phase LC or SFC depending on the purity of the original sample and the amount of purified material needed. Many samples from medicinal chemists are crude mixtures containing starting materials, by-products, and residual catalysts, which can interfere with the isolation process. Removing these impurities by reversed-phase LC is generally preferred because of its capacity for handling compounds of a wide polarity range (5). If separation by reversed-phase LC is not successful, then achiral SFC may be the next screening step (7). In general, SFC is preferred as the final purification step when larger amounts of COI are required (such as >300 mg) or for those that are unstable or insoluble in the presence of water.
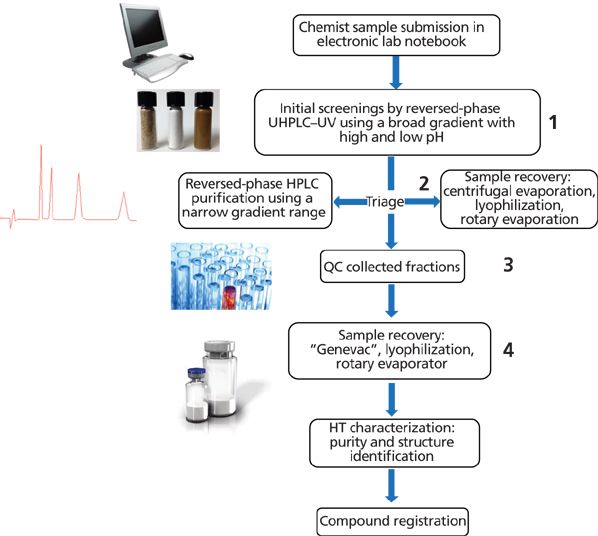
Figure 3: The high-throughput purification workflow in a centralized analytical laboratory supporting discovery chemistry in a pharmaceutical company. The four typical separations steps in the workflow are as follows: initial reversed-phase LC–m –MS screen; reversed-phase LC or SFC preparative purification; quality control of collected fractions; and sample recovery.
Rapid Initial Screening Using Reversed-Phase LC
All purifications in our laboratory start with an initial ultrahigh-pressure LC (UHPLC) screening step. Exceptions are COI that are unstable in water or single compounds requiring immediate chiral purity assessment where SFC is preferred. We typically screen using reversed-phase LC–ultraviolet (UV)–mass spectrometry (MS) with fast, broad gradients using a short sub-2-μm C18 column with both acidic and basic mobile phases as the weak solvents (mobile-phase A) (4,5). These 2-min screening methods allow quick assessment of sample purity, peak confirmation (MS), and subsequent selection of optimal preparative reversed-phase LC parameters. Our default mobile-phase additives are 0.1% formic acid and 0.1% ammonia for high MS sensitivity and low volatility for ease of elimination in the sample recovery step (8–10). Using either of these mobile-phase conditions, we usually are able to obtain baseline resolution for most samples. Trifluoroacetic acid, triethylamine, and the ammonium salts of formate, acetate, or carbonate are less desirable for volatility and recovery reasons (5,8). An example of the initial screening step is shown later in the case study.
Rapid Achiral and Chiral Screening Using SFC
For achiral SFC separations, normal phase columns packed with silica, cyano, diol, and 2-ethylpyridine bonded phases are the most popular, though reversed-phase columns such as C18 have found some success (11,12). For chiral separations, columns packed with derivatized polysaccharides (coated or immobilized) on silica support or Pirkle-type chiral stationary phases are the most common (4,12,13).
Our approach to achiral and chiral SFC method development is to screen the compounds rapidly using short gradients. For achiral SFC, we typically screen six 50 mm × 3.0 mm columns packed with sub-2-μm particles (when available), with a flow rate of 1.5 mL/ min, a run time of 1.5 min and a 5–60% B gradient. For chiral SFC, we screen six 50 mm × 4.6 mm columns packed with 3-μm particles, with a flow rate of 4 mL/min, a run time of 2.5 min, and a 5–60% B gradient. We selected a combination of screening phases that appears to work well for our type of compounds based on an internal study (14). This method development process usually allows scale-up to preparative SFC using isocratic or gradient conditions. An example of this screening process is discussed later in the case study.
Preparative Reversed-Phase LC
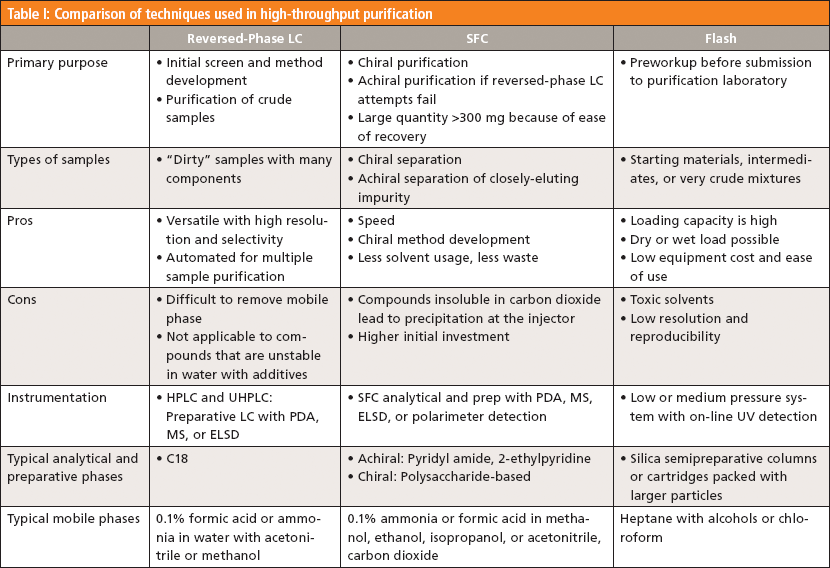
The goal of preparative chromatography, whether by LC or SFC, is to obtain purified materials of sufficient quality (purity, form) and quantity (yield) in a reasonable time (for example, 10–20 min). Contrary to the predominance of flash chromatography by normal-phase LC in organic chemistry laboratories, reversed-phase LC is commonly used in our laboratory because of its higher reproducibility and resolution, MS-compatibility, and high likelihood for recovery of desired materials in a solid powder form (4–6). Preparative normal-phase LC is mostly supplanted by the "greener" SFC in centralized laboratories because of the higher speed and faster sample recovery (7,13). Table I summarizes comparative characteristics and parameters used in reversed-phase LC, SFC, and normal-phase LC. Since concepts and practices of preparative LC and SFC can be found in many books (6,7,15) and articles (16–18) in great detail, we will only summarize those for high-throughput purification applications.
Columns packed with octadecylsilane (C18) bonded phase remain the universal columns of choice in reversed-phase LC because of their reliability and sample capacity (5). Newer packing materials such as hybrids (for example, Waters BEH or Phenomenex Gemini-NX) are popular because of their compatibility with high-pH mobile phases, allowing improved retention and better peak shapes for basic pharmaceuticals (5). Column inner diameters (21.2–30 mm i.d) are dictated by sample amounts and the flow rate ranges of the preparative equipment at hand. Column lengths tend to range from 50 to 100 mm for speedy separations, although longer column lengths may be needed to provide higher resolution. Common particle sizes used are 5 and 10 μm for cost and pressure considerations. Mobile phases (water and acetonitrile [or methanol]) with additives (0.1% formic acid or ammonia) are similar to those used in initial screening. Flow rates are geometrically scaled to preparative column diameters (5). We typically use narrower gradient ranges to increase the resolution around the COI. For instance, four standard ranges are typically used in our laboratory (5–50% B, 20–60% B, 30–70% B, or 40–80% B) depending on the hydrophobicity of the COI (19), which can be estimated from initial screens.
Diluents of strong solubilizing power such as dimethylformamide or DMSO are typically used to dissolve the sample. Low-solubility compounds may require larger injection volumes or multiple injections. Sample concentrations are kept high when possible to minimize injection volumes or the number of injections (15). Common detection modes where signals can be used to trigger automated fraction collection are UV, MS, (termed UV-directed or MS-directed purification, respectively), and evaporative light scattering detection (ELSD). While UV-directed systems are simple and less expensive, they are limited to chromophoric compounds. MS-directed systems are versatile and preferred for purification of compound library collections. ELSD-directed collections are typically used for purification of natural products or nonchromophoric products. In our laboratory, we use both UV- and MS-directed purification systems with automated fraction collection and subsequent QC. Fractions containing the COI are then recombined for sample recovery. This semiautomatic approach allows us more flexibility to handle a variety of complex samples and amounts.
Preparative SFC
The last decade witnessed significant improvements in the reliability and performance of SFC instrumentation. The benefits of higher speed, resolution, and easier sample recovery using environmentally friendly solvents (carbon dioxide) are well publicized and recognized (7,18). The principles, practices, and applications of SFC are available in numerous books and articles (7,17,18,20).
SFC operates in the normal-phase mode with carbon dioxide serving as the weak mobile phase with increasing concentration of cosolvents such as methanol, ethanol, or isopropanol. The cosolvents are spiked with acidic or basic additives to keep the analytes in non-ionized forms to prevent sample adsorption on these polar stationary phases (4,13). Our laboratory pioneered the use of ammonia in place of triethylamine or diethylamine in SFC (8), which yields similar separations and allows easier recovery of the COI as a free base. Earlier concerns of reduced lifetime of columns have not been observed.
Sample precipitation in the injector, which can cause equipment failure, is a real issue in preparative SFC. While a strong diluent such as DMSO can be used for achiral SFC, we prefer to use methanol as the diluent to match the mobile phase most often used in SFC. Sample concentrations are scaled back 2–3-fold from those used in reversed-phase LC to minimize potential problems. Yields and sample throughput are often increased with "stacked" injections whereby injections are stacked to maximize efficiency (an injection mode in which the next sample is injected before the complete elution of the current sample). Achiral and chiral method development strategies in SFC and column selection are published elsewhere (21–23).
Post-Purification QC Using UHPLC–UV–MS
In our laboratory, post purification QC by UHPLC of the collected fractions is performed concurrently during purification or after the purification is complete. Additional analysis of the fractions by UHPLC under opposite pH mobile phase conditions provides an orthogonal purity assessment and decreases the chances of missing coeluted impurities. A more detailed description of high-throughput structure confirmation and quantitation will be discussed in the next installment. Results from these quick QC analyses of the fractions collected allow for a rational combination of all COI-containing fractions into one container for improving sample recovery and optimizing yield or purity.
Sample Recovery
A vital step in purification of a COI is the removal of all solvents and additives to yield a pure powder that is amenable to weighing, distribution, and long-term storage. Solvent removal can be achieved by centrifugal evaporation (that is, Genevac), lyophilization (freeze-drying) (24,25), or rotary evaporation. Occasionally, a residual oil instead of a solid is obtained and must be submitted as such. Newer evaporation approaches using trapping columns may be able to address this issue (26).
Centrifugal evaporation systems are popular because of their efficiency, programmability, and the convenience of using the same vials from fraction collection. These type of systems should not be used for thermally labile or semivolatile compounds. Freeze dryers are best suited for removing aqueous solvents by sublimation at room temperature. The downsides are longer evaporation time and potential equipment damage from exposure to organic solvents, if present in large quantities. Rotary evaporators are efficient for removing organic and aqueous solvents, but do not usually lead to dry powders. We use rotary evaporators to reduce large volumes of solvents prior to the final drying steps in a centrifugal evaporator or lyophilizer.
A Case Study Illustrating the Purification Strategy: Isolation of R- and S-Propranolol
To illustrate the workflow in high-throughput purification as illustrated in Figure 3, we present here a case study on the isolation of R- and S-propranolol from a drug mixture. The sample was a mixture of commercial drugs consisting of 50 mg of racemic propranolol, 20 mg of bupivacaine HCl, 20 mg of naproxen, and 50 mg of warfarin. This sample was used to simulate a more complex purification scenario requiring a reversed-phase LC cleanup step before chiral SFC to yield purified enantiomers. The sample was dissolved in 1.4 mL of dimethylformamide at 100 mg/mL. This sample solution was further diluted 1000:1 in methanol to ~0.1 mg/mL for initial screening.
Step 1: Initial UHPLC–UV–MS Screening
The initial screening of the sample was performed under typical high-throughput screening UHPLC conditions with a broad gradient using a 30 mm × 2.1 mm i.d. sub-2-μm C18 column. Both acidic and basic mobile phases A were used consecutively in separate gradient separations using a standard protocol as shown in Figure 4 (first and second chromatograms). Note that basic mobile phase A yielded an acceptable separation of all four components. Under high-pH conditions, the acidic naproxen and warfarin are ionized and have lower retention, while the basic propranolol and bupivacaine are un-ionized and are strongly retained (5). The initial screens provided quick assessments of sample purity and impurity profile, which is useful information for devising a purification strategy. Results indicated the need for a two-stage purification process: reversed-phase LC to isolate the COI from other components and a chiral SFC purification step to obtain purified enantiomers.
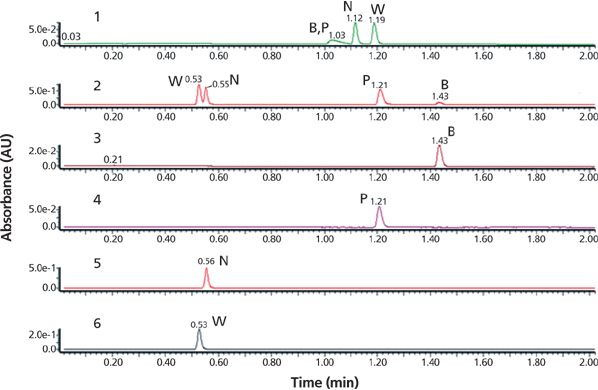
Figure 4: Chromatograms showing the results of step 1 of initial sample screening of the case study sample. The sample consisted of bupivacaine (B), propranolol (P, COI), naproxen (N), and warfarin (W), which were subjected to UHPLC–UV–MS screening using either acidic (chromatogram 1) or basic (chromatogram 2) mobile phases, A, with an acetonitrile gradient, B. UHPLC column: 30 mm × 2.1 mm, 1.7-μm Waters Acquity BEH C18 (Waters) at 35 °C; mobile-phase A: 0.1% (v/v) formic acid or 0.1% (v/v) ammonium hydroxide in water; mobile-phase B: acetonitrile; linear gradient: 5–100% B in 1.5 min; flow rate: 0.8 mL/min; detection: UV absorbance at 254 nm; temperature: 35 °C; injection volume: 1 μL at 0.1 mg/mL. The use of 0.1% formic acid mobile phase shows poor resolution of the four components, chromatogram 1. The use of 0.1% ammonium hydroxide mobile phase shows excellent resolution of all four components, chromatogram 2. Individual standards of bupivacaine, propranolol, naproxen, and warfarin analyzed under basic mobile phase conditions are shown in chromatograms 3–6, respectively.
Step 2a: Preparative Reversed-Phase LC Purification
A preparative reversed-phase LC–UV isolation step was used to isolate the COI from the sample mixture. Figure 5 shows the chromatogram and operating details of a single injection (1.4 mL) of the entire sample dissolved in dimethylformamide. Note that while narrower gradient ranges are typically used in this step to maximize the resolution around the COI (13), this was not the case for this study because the sample contains components of widely ranging hydrophobicity. Fraction collection was triggered by the UV signal. The collected fractions of interest were pooled and subsequently analyzed (QC) in step 3a. The column dimensions (50 mm × 30.0 mm) were dictated by the purification amount (140 mg) with flow rate (60 mL/min) scaled to the column size.
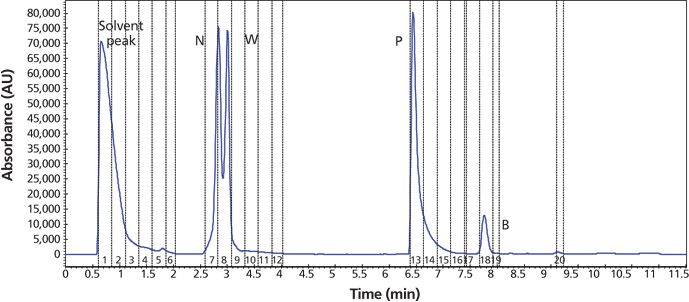
Figure 5: Reversed-phase LC–UV preparative chromatogram showing step 2a purification of the sample to isolate the COI (propranolol) from other components. HPLC column: 50 mm × 30.0 mm, 5-μm Phenomenex Gemini-NX; mobile-phase A: water with 0.1% ammonium hydroxide; mobile-phase B: acetonitrile; linear gradient: 2–80% B in 10 min; flow rate: 60 mL/min; detection: UV absorbance at 220 nm; temperature: ambient; injection volume: 1.4 mL containing the entire sample of 140 mg in dimethylformamide. Fraction collection time points triggered by the UV signal are labeled with vertical dashed lines: 0.5–1.25 min, the solvent peak (dimethylformamide); 2.5–3 min naproxen and warfarin; 6.4–7.2 min, propranolol; 7.7–7.9 min, bupivacaine. Fractions 7–8 and 13–15 were pooled and analyzed. The isolated COI, propranolol, was subjected to further SFC chiral purification to obtain pure enantiomers.
Step 3a: QC of Collected Fractions
This reversed-phase LC–UV–MS QC step was used to confirm the identity of the appropriate fractions using UHPLC conditions similar to those used in the initial screening (Figure 6). If the purity and identity (%area at 220 nm and MS) were deemed acceptable, the pooled fractions were evaporated to yield materials for chiral SFC. A total recovery weight of the R- and S-propranolol from this step was 44.7 mg after rotary evaporation of fractions 13–15 shown in Figure 5.
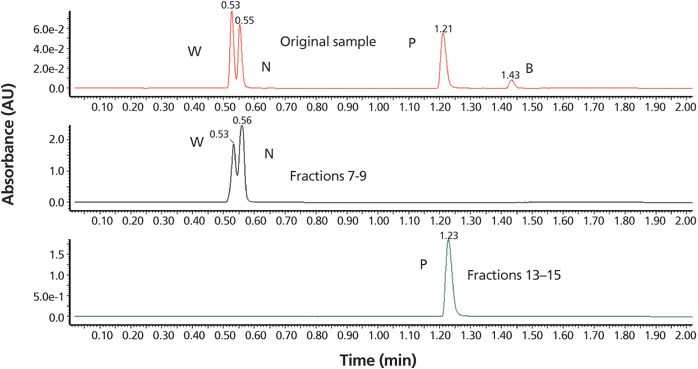
Figure 6: Chromatogram showing step 3a of the workflow in the analysis of collected and pooled fractions using UHPLC (reversed-phase LC–UV–MS) with the initial screening conditions of Figure 4, chromatogram 2. UV detection was set to 220 nm and an injection volume of 1 μL was used. Chromatograms: upper, original sample; middle, fractions 7–9; and lower, fractions 13–15 (containing the racemic COI, R- and S-propranolol).
Step 2b: Chiral SFC Purification
Chiral method development using an automated column-mobile phase screening system was performed (Waters Acquity UPC2 SFC system). The isolated propranolol racemic sample was dissolved in 2 mL of methanol (22.4 mg/mL) and then further diluted to 0.1 mg/mL. The diluted sample was screened on six chiral columns under standard gradient conditions with the results shown in Figure 7. This screen was completed in less than 30 min and led to the selection of Phenomenex Lux Cellulose-1 column for the purification step.
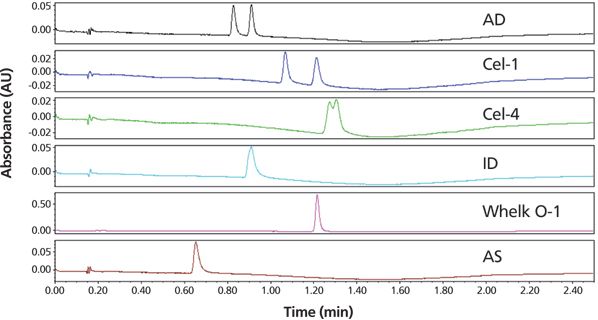
Figure 7: Chromatograms showing the chiral column screening for separating R- and S-propranolol using an automated chiral SFC analytical screening system (Waters Acquity UPC2). SFC conditions: columns: Chiralpak AD, Lux Cellulose-1, Lux Cellulose-4, Chiralpak ID, Regis Whelk-O 1 (S,S), Chiralpak AS; all columns were 50 mm × 4.6 mm, 3-μm particle size except for Whelk-O 1, which has a particle size of 3.5 μm; column temperature: 40 °C; mobile-phase A: carbon dioxide; mobile-phase B: 0.1% ammonium hydroxide in methanol; linear gradient: 5–60% B in 1.8 min; run time: 2.5 min; flow rate: 4 mL/min; back-pressure regulator: set to 120 bar; detection: UV absorbance at 220 nm; injection volume: 1 μL of 0.1 mg/mL solution.
Preparative chiral SFC-UV purification was performed on a PIC-100 SFC system using a 150 mm × 21.2 mm, 5-μm Phenomenex Lux Cellulose-1 column (Figure 8) under optimized isocratic conditions. An initial exploratory injection was made to identify the elution time and loadability of the sample. To increase throughput, stacked injections were used with a 2-min cycle time, leading to a total run time of 25 min. Fractions containing methanolic solutions of each purified enantiomer were transferred to round-bottomed flasks and rotary evaporated to remove the 120 and 170 mL of methanol containing the respective stereoisomers. Each enantiomer was then transferred to a pretared vial using a 2:3 (v/v) mixture of water and acetonitrile for subsequent sample recovery to obtain solid powder.
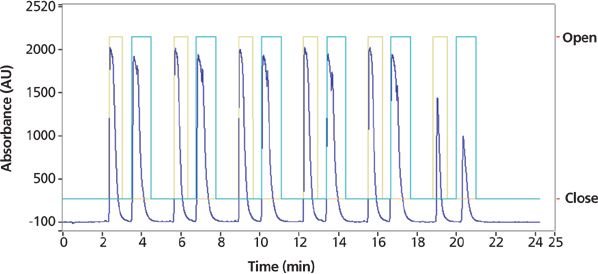
Figure 8: Preparative SFC chromatograms of the final step 2b purification showing a sequence of six consecutive staggered injections of the racemic propranolol sample isolated in the final step 2a purification to yield purified enantiomers on a PIC-100 system. 44.7 mg of the isolated propranolol was dissolved in 1.5 mL of methanol diluent and a "scout'' injection of 0.2 mL was made to identify retention times and loadability. Injections (0.2 mL) of the sample were made every 2 min under optimized isocratic conditions. Preparative SFC conditions: column: 150 mm × 21.2 mm, 5-μm Phenomenex Lux Cellulose-1; mobile-phase A: carbon dioxide; mobile-phase B: 0.1% ammonium hydroxide in methanol; isocratic run: 25% B for 25 min; flow rate: 70 mL/min; back-pressure regulator: set to 100 bar; temperature: 40 °C; detection: UV absorbance at 220 nm.
Step 4: Sample Recovery
The vials were frozen on dry ice and placed in a Genevac HT-4 evaporator. Lyophilization or freeze drying is also possible, although centrifugal evaporation is typically preferred for quicker drying. The recovery for each enantiomer was 20.1 mg and 22.1 mg for peaks 1 and 2, respectively.
Step 3b: Final SFC QC
The enantiomeric purity was confirmed using a Waters Acquity UPC2 system and an isocratic method with a mobile phase of 20% methanol with 0.1% ammonium hydroxide on a Phenomenex Lux Cellulose-1 column (Figure 9). Isolated samples were identified as R- or S-propranolol respectively by retention time matching with genuine reference standards.
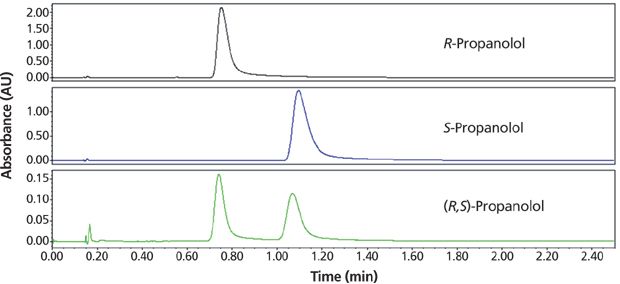
Figure 9: Chromatograms of the final step 3b of QC or purity analysis of R- and S-propranolol using a Waters UPC2 analytical SFC system. A 0.1-mg/mL solution of each enantiomer was prepared by weighing out a sample and dissolving it in methanol. SFC conditions: column: 50 mm × 4.6 mm, 3-μm Phenomenex Lux Cellulose-1; mobile-phase A: carbon dioxide; mobile-phase B: 0.1% ammonium hydroxide in methanol; isocratic run: 20% B for 2.5 min; flow rate: 4 mL/min; back-pressure regulator: set to 120 bar; detection: UV absorbance at 220 nm; temperature: 40 °C; injection volume: 2 μL. The top two chromatograms result from analyses of the isolated pure propranolol enantiomers and the bottom chromatogram is from analysis of the original propranolol racemate.
The Overall Purification Process
The entire purification process took two days with an overall recovery of >80% yielding a purity of COI that exceeded our general criterion of >95% for both chemical and chiral purity. The next step is high-throughput characterization, which will be described in the next installment of this series. This case study describes our purification processes and serves to illustrate our parameter selection rationales using chromatographic principles to balance sample throughput with quality, scale, and other requirements.
Status and Trends in High-Throughput Purification
The emergence of SFC as a preferred technology for chiral analysis and purification is perhaps the most notable trend (7,8,20,27). Further development of newer instruments combining supercritical fluid extraction (SFE) with SFC may prove useful for on-line analysis of labile samples extracted from complex matrices (28). Two-dimensional (2D) systems combining two orthogonal separation modes (for example, reversed-phase LC-SFC) may also offer quicker and more automated approaches for samples from complex matrices (29,30).
The adaptation of automation in solid and liquid handling improves the reliability and productivity of the purification process (16,17). Automation would impact most in areas such as fraction pooling and QC or weighing of powder samples, particularly in laboratories with more predictable workflow in handling library purification. In our laboratory, we use a semiautomated approach for specific processes in the workflow (for example, screening, chiral method development, analysis of collected COI, and drying) to maintain flexibility to handle diverse sample types or scales and purity requirements.
The steps involved from sample isolation and recovery to production of a solid powder amendable to accurate weighing is labor-intensive, time consuming, and somewhat unpredictable at times. We have found lyophilization and centrifugal vacuum evaporation to be the most useful. New approaches (such as Shimadzu's Crude2Pure system) with automated evaporation from trapping resins may offer another viable alternative (26).
Finally, new advances in column technologies will continue to impact this application area. We have described the routine use of sub-2-μm particles, hybrids, immobilized polysaccharides, and new SFC bonded phases in our examples. The emerging dominance of superficially porous particles (SPP) is likely to impact high-throughput screening method development and sample analysis in the analytical scale (31), although SPPs are less likely to be implemented in preparative-scale separations because of cost factors. Newer and more effective columns for SFC and chiral separations are also expected to continue to be active research areas in the foreseeable future.
Conclusion
In this installment, we discussed modern technologies and processes used in high-throughput purification that support small-molecule drug discovery. We also described the workflow taking place in a centralized group using rapid initial screening, reversed-phase LC or SFC purification, quality control analysis of resulting fractions, and sample recovery. A case study, in which two purified enantiomers of propranolol from a drug mixture were isolated and purified, illustrated the principles and rationales used to achieve the end goal of delivering materials of sufficient quality and quantity in a reasonable time span.
Acknowledgments
The authors would like to thank Chris Hamman, Dr. Kelly Zhang, and Snahel Patel of Genentech; Dr. Szabolcs Fekete of the University of Geneva; William Farrell and Christine Aurigemma of Pfizer; Larry Miller of Amgen; Professor Larry Taylor of Virginia Tech; and Dr. Tom Waeghe of MAC-MOD Analytical for their editorial inputs and suggestions.
References
(1) Drug Discovery and Development: Technology in Transition, 2nd Edition, R.G. Hill and H.P. Rang, Eds. (Churchill Livingston, Edinburgh, Scotland, 2012).
(2) Drug Discovery: Practices, Processes, and Perspectives, 1st Edition, J.J. Li and E.J. Corey, Eds. (Wiley, Hoboken, New Jersey, 2013).
(3) T. Nograd and D.F. Weaver, Medicinal Chemistry: A Molecular and Biochemical Approach, 3rd Edition (Oxford University Press, New York, New York, 2005).
(4) Handbook of Pharmaceutical Analysis by HPLC, S. Ahuja and M.W. Dong, Eds. (Elsevier/Academic Press, Amsterdam, Netherlands, 2005).
(5) M.W. Dong, Modern HPLC for Practicing Scientists (Wiley, Hoboken, New Jersey, 2006), Chapters 2, 4, and 8.
(6) D.B. Kassel, in HPLC for Pharmaceutical Scientists, Y.V. Kazakevich and R LoBrutto, Eds. (Wiley, Hoboken, New Jersey, 2007), pp. 535–575.
(7) Supercritical Fluid Chromatography: Advances and Applications in Pharmaceutical Analysis, G.K. Webster, Ed. (CRC Press, Taylor and Francis, Boca Raton, Florida, 2014).
(8) C. Hamman, D.E. Schmidt, M. Wong, and M. Hayes, J. Chromatogr. A1218, 7886–7894 (2011).
(9) Z. Wu, W. Gao, M.A. Phelps, D. Wu, D.D. Miller, and J.T. Dalton, Anal. Chem.76, 839–847 (2004).
(10) M.C. Garcia, J. Chromatogr B.825(2), 111–123 (2005).
(11) M. Ashraf- Khorassani, L.T. Taylor, and E. Seest, J. Chromatogr. A 1229, 237–248 (2012).
(12) T. Berger and B. Berger, LCGC North Am. 28(5), 344–357 (2010).
(13) Preparative Enantioselective Chromatography, G. Cox, Ed. (Wiley-Blackwell, Hoboken, New Jersey, 2005).
(14) C. Hamman, M. Wong, I. Aliagas, D.F. Ortwine, J. Pease, D.E. Schmidt, and J. Victorino, J. Chromatogr A.1305, 310–319 (2013).
(15) D.A. Wellings, A Practical Handbook of Preparative HPLC (Elsevier, Oxford, United Kingdom, 2006).
(16) W. Goetzinger, X. Zhang, G. Bi, M. Towle, D. Cherrak, and J.N. Kyranos, Int. J. Mass Spec.238, 153–162. (2004).
(17) M. Liu, K. Chen, D. Christian, T. Fatima, N. Pissarnitski, E. Streckfuss, C. Zhang, L. Xia, S. Borges, Z. Shi, P. Vachal, J. Tata, and J. Athanasopoulos, ACS Comb. Science14, 51–59 (2012).
(18) L.T. Taylor, J. Supercritical Fluids47, 566–573 (2009).
(19) M.W. Dong, LCGC North Am.31(8), 612–621 (2013).
(20) Supercritical Fluid Methods and Protocols (Methods in Biotechnology), J.R. William and A.R. Clifford, Eds. (Humana Press, New York, New York, 2000).
(21) D. Speybrouck, D. Corens, and J.M. Argoullon, Current Topics in Med. Chem. 12, 1250–1263 (2012).
(22) N. Hicks, V.S. Sharp, and J. Stafford, LCGC North Am. 31(8), 622–628, (2013).
(23) C. Hamman, M. Wong, M. Hayes, and P. Gibbons, J. Chromatogr. A1218(22), 3629–36 (2011).
(24) Freeze-Drying/Lyophilization of Pharmaceutical and Biological Products, 3rd Edition, L. Rey and J.C. May, Eds. (CRC Press, Boca Raton, Florida, 2010).
(25) J.C. Kasper, G. Winter, and W. Friess, European J. Pharm. and Biopharm. 85, 162–169 (2013).
(26) M.W. Dong, LCGC North Am.32(4), 270–279, (2014).
(27) K.W. Phinney, L.C. Sander, and S.A. Wise, Anal. Chem.70, 2331–2335 (1998).
(28) T. Uchikata, A. Matsubara, E. Fukusaki, and T. Bamba, J. Chromatogr. A1260, 69–75 (2012).
(29) C.J. Welch, M. Biba, J.R. Gouker, G. Kath, P. Augustine, and P. Hosek, Chirality19, 184–189 (2007).
(30) M. Ventura, W. Farrell, C. Aurigemma, K. Tivel, M. Greig, J. Wheatley, A. Yanovsky, K.E. Milgram, D. Dalesandro, R. DeGuzman, P. Tran, L. Nguyen, L. Chung, O. Gron, and C.A. Koch, J. Chromatogr. A1036, 7–13 (2004).
(31) S. Fekete, D. Guillarme, and M.W. Dong, LCGC North Am. 32(6), 420–433 (2014).
Joseph H. Pease leads the Analytical Chemistry & Purification Department in Small Molecule Discovery Chemistry at Genentech, Inc. He earned a BS degree in Chemistry from U.C. Davis and a PhD in Biophysical Chemistry from U. C. Berkeley. Joe joined Syntex in 1990 as a research scientist specializing in NMR and structure-based drug discovery. After Roche acquired Syntex in 1994 to form Roche Bioscience, he stayed with the company until its closure in 2010 and then joined Genentech in 2011. During his career, Dr. Pease has held leadership positions in discovery research, preclinical development, and informatics.

Joseph H. Pease
Brent Murphy has a BS in Biochemistry and Molecular Biology from U. C. Santa Cruz. He is a senior research associate in the Analytical Chemistry & Purification department in Small Molecule Discovery Chemistry at Genentech, Inc. Over his 13 years in the laboratory, he has gained experience with a variety of projects from purification of natural products and peptides to chiral separations of small molecules. Current interests include large-scale preparative SFC and SFE. His prior job experience was with the Lokey Group at U. C. Santa Cruz, Theravance, and Amgen.

Brent Murphy
Mengling Wong earned a BS in Biochemistry from U. C. Davis. She is a senior research associate in the Analytical Chemistry & Purification department in Small Molecule Discovery Chemistry at Genentech, Inc. Over her 14 years at Genentech, she has acquired analytical and purification skills in the areas of peptides and small molecules. Her interests are in SFC and HPLC technologies and their applications in separating early discovery chiral and achiral molecules. Prior to Genentech, she was at Keystone Division of Biosource International as a laboratory supervisor in the synthesis and separations of oligonucleotides.

Mengling Wong
Michael W. Dong is a principal in MWD Consulting focusing on training and consulting services in HPLC, UHPLC, pharmaceutical analysis, and drug quality. He was formerly Senior Scientist at Genentech, Small Molecule Analytical Chemistry and QC, Research Director at Synomics Pharma, Research Fellow at Purdue Pharma, and Senior Staff Scientist at Applied Biosystems/PerkinElmer. He holds a PhD in Analytical Chemistry from City University of New York, and a certificate in Biotechnology at University of California, Santa Cruz. He has more than 100 publications and a best-seller in chromatography. He is an editorial advisory board member of LCGC North America.

Michael W. Dong
Direct correspondence to: michaelwdong2015@gmail.com

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).















