GC Temperature Programming—10 Things You Absolutely Need to Know
LCGC North America
Temperature affects not only retention but also relative retention in gas chromatography (GC) and therefore, when we change temperature, we also change the selectivity of the separation. This is true as we alter the isothermal separation temperature, but also as we change the slope of the temperature program gradient.
1. Temperature affects not only retention but also relative retention in gas chromatography (GC) and therefore, when we change temperature, we also change the selectivity of the separation. This is true as we alter the isothermal separation temperature, but also as we change the slope of the temperature program gradient.
2. An increase of around 30 °C in oven temperature will reduce retention time by 50%.
3. Use the method shown in Figure 1 to "screen" samples.
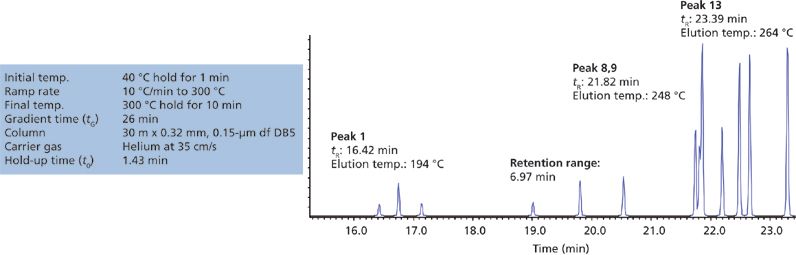
Figure 1: Extract of chromatogram showing all pesticide analyte peaks eluted from a river water extract, analyzed under the screening conditions shown.
4. If the peaks are eluted within a "window" of less than 7 min (more accurately tg/4) then isocratic analysis may be possible (6.97 in our example, so isothermal analysis may be possible).
5. To approximate the required isothermal temperature for the separation, calculate the temperature at which the last analyte of interest is eluted and subtract 45 °C (239 °C in our example).
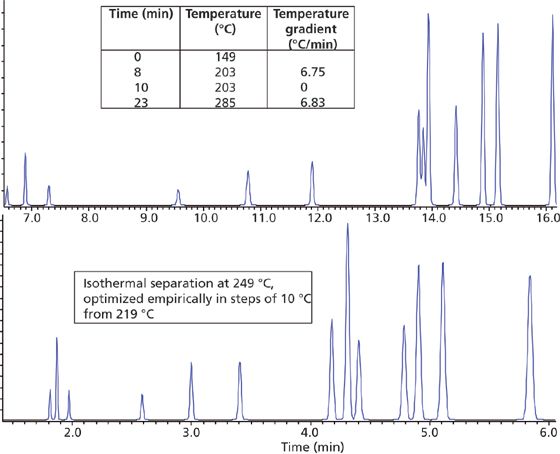
Figure 2: Optimum separations derived from a thermal gradient analysis (top) and an isothermal analysis (bottom).
6. To optimize the separation, alter the isothermal temperature in steps of 10 °C, within a range of ±50 °C. If a suitable separation is not obtained a temperature gradient should be used.
7. For splitless injection, the initial oven temperature should be 20 °C below the boiling point of the sample solvent and an initial hold time of 30 s should be used initially. For split injection, start with an oven temperature 45 °C lower than the elution temperature of the first peak from the screening chromatogram (149 °C in our exercise). For poorly resolved, early eluted peaks, decrease initial temperature rather than adding an initial isothermal hold unless the initial oven temperature is more than 30 °C below the boiling point of the sample solvent.
8. The optimum ramp rate for any separation can be estimated as 10 °C per hold-up time (7 °C/min in our screening example).
9. If a suitable gradient slope cannot be obtained to separate compounds eluted in the middle of the temperature gradient, insert a mid-ramp isothermal section at 45 °C below the elution temperature of the critical pair (203 °C to help separate peaks 8 and 9 in our screening example). Empirically determine the length required for the hold (start with a 1-min hold) and then resume the gradient at the same slope as before.
10. Set the final temperature at 20 °C above the elution temperature of the last analyte in the screen, but bear in mind that a higher temperature "burn" period may be required to elute high-boiling matrix components.

Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
Using GC-MS to Measure Improvement Efforts to TNT-Contaminated Soil
April 29th 2025Researchers developing a plant microbial consortium that can repair in-situ high concentration TNT (1434 mg/kg) contaminated soil, as well as overcome the limitations of previous studies that only focused on simulated pollution, used untargeted metabolone gas chromatography-mass spectrometry (GC-MS) to measure their success.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.
Potential Obstacles in Chromatographic Analyses Distinguishing Marijuana from Hemp
April 28th 2025LCGC International's April series for National Cannabis Awareness Month concludes with a discussion with Walter B. Wilson from the National Institute of Standard and Technology’s (NIST’s) Chemical Sciences Division regarding recent research his team conducted investigating chromatographic interferences that can potentially inflate the levels of Δ9-THC in Cannabis sativa plant samples, and possible solutions to avoid this problem.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












