Current State of Superficially Porous Particle Technology in Liquid Chromatography
LCGC North America
The use of superficially porous particles in the manufacture of high performance liquid chromatography (HPLC) columns has become prominent in recent years. Over the course of the past decade most major manufacturers have built column lines around the technology.
The use of superficially porous particles in the manufacture of high performance liquid chromatography (HPLC) columns has become prominent in recent years. Over the course of the past decade most major manufacturers have built column lines around the technology. At the recent Pittcon conference in New Orleans, Louisiana, a large number of oral and poster presentations centered on the current research and advances using superficially porous particles. This installment aims to provide some highlights of these recent trends.
A major goal in high performance liquid chromatography (HPLC) over the past decade has been to develop particle technologies that provide enhanced separation efficiency. The trend began with the introduction of sub-2-μm particles along with advances in HPLC instrumentation. Modern superficially porous particles (SPPs) for use with small-molecule separations were introduced in 2006 by Advanced Materials Technologies. The advantage of SPPs was that the technology provided nearly similar efficiencies to the sub-2-μm particles, but at a lower cost of back pressure. The lower back pressure then allowed the technology to be used across a wider array of HPLC systems.
Since its introduction, SPP technology has been embraced by most major column manufacturers and HPLC practitioners. At Pittcon 2015 in New Orleans, Louisiana, it was clear from the number of presentations that active research and development is continuing in this realm. A few major trends were observed:
- expansion of particle sizes and pore geometries,
- additional chemical surface modifications, and
- application beyond small molecule reversed-phase analyses.
This installment is intended to discuss recent observed trends in SPP development. For more background on the technology, readers are referred to several reviews published in recent years (1–3).
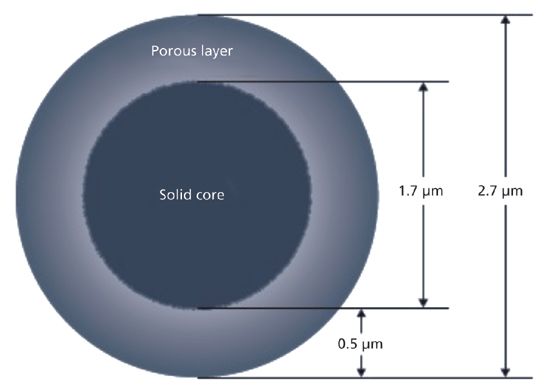
Figure 1: Representation of superficially porous particle technology showing the general architecture of modern superficially porous particles as introduced by Kirkland (Advanced Materials Technology) in 2006. (Adapted with permission from www.hplc.eu/halo.htm.)
Superficially Porous Particles
Superficially porous particles (also known as solid core, core–shell, or Fused-Core particles) exhibit a solid core surrounded by a thin layer of porous material. The SPP architecture was initially designed to improve mass transfer kinetics over fully porous particles (FPPs); however, further research has revealed that the increased efficiencies observed in small-molecule applications is largely because of improvements in both eddy diffusion (the A term in the van Deemter equation) and longitudinal diffusion (the B term) (4). The improvement in eddy dispersion has been attributed to the inherent low particle size distribution resulting from the SPP construction process and on the roughness of the SPP surface (5). These features result in a higher packing density than observed with FPP phases. The presence of the solid core has a positive impact on the longitudinal diffusion and has been shown to reduce the longitudinal diffusion by approximately 30% over fully porous particles (6).
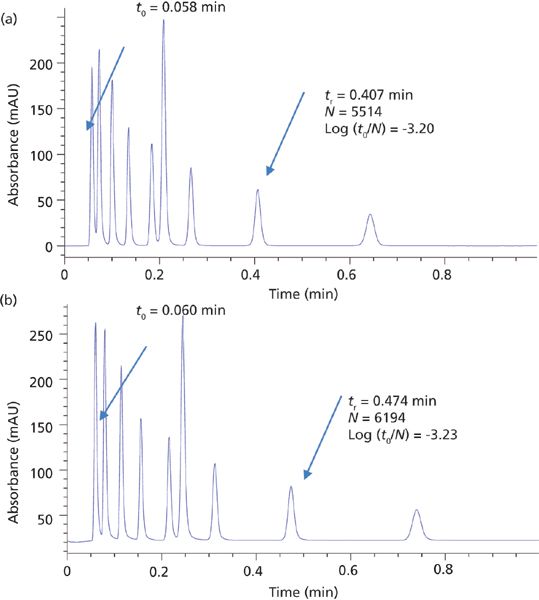
Figure 2: Ultrafast separations obtained using (a) superficially porous and (b) totally porous particle 50-mm columns. (a) Column: 50 mm × 2.1 mm, 2.7-μm Poroshell 120 EC-C18; mobile phase: 56:44 acetonitrile–water; flow rate: 1.94 mL/min; temperature: 40 °C; pressure: 549 bar. (b) Column: 50 mm × 2.1 mm, 1.8-μm Zorbax RRHD Eclipse Plus C18; mobile phase: 59:41 acetonitrile–water; flow rate: 1.82 mL/min; temperature: 40 °C; pressure: 1003 bar. (Courtesy of Agilent Technologies.)
Recent Trends in Superficially Porous Particle Design
Expansion of Particle Sizes and Pore Geometries
The initial offering from Advanced Materials Technology in 2006 consisted of a 1.7-μm solid core and a 0.5-μm outer shell with a pore size of 90 Å (see Figure 1). Since that time most column manufacturers have adopted some form of SPP technology. As demonstrated in Figure 2, SPPs at 2.7 μm can provide chromatographic efficiencies nearly similar to smaller sub-2-μm FPPs, but with about half the back-pressure burden. A listing of some of the typical brands and their SPP offerings is provided in Table I. It is clear from this list that SPP introductions have grown to cover a large array of particle sizes and surface chemistries. Several of the manufacturers in Table I have introduced 2.0-μm or sub-2-μm versions of the SPP technology. The smaller particles offer increased efficiency over 2.7-μm and larger particles and are generally used with ultrahigh-pressure liquid chromatography (UHPLC) instrumentation. The same trends in lower optimum plate heights and flatter C terms with smaller overall particle size are observed with SPPs as previously was achieved with fully porous particles. Some of these manufacturers have also introduced larger (4–5 μm) shell particles. The larger particle sizes provide practitioners with even less of a back-pressure burden than the 2.7-μm particles and allow easier transfer of methods based on common 5-μm fully porous particles. Studies have shown that the larger sized SPP technology still provides improved efficiency and flatter C terms than similarly sized fully porous particles (7,8). Figure 3 shows a comparison of the van Deemter plots (H versus u0) for 5-μm SPPs and 5-μm TPPs.
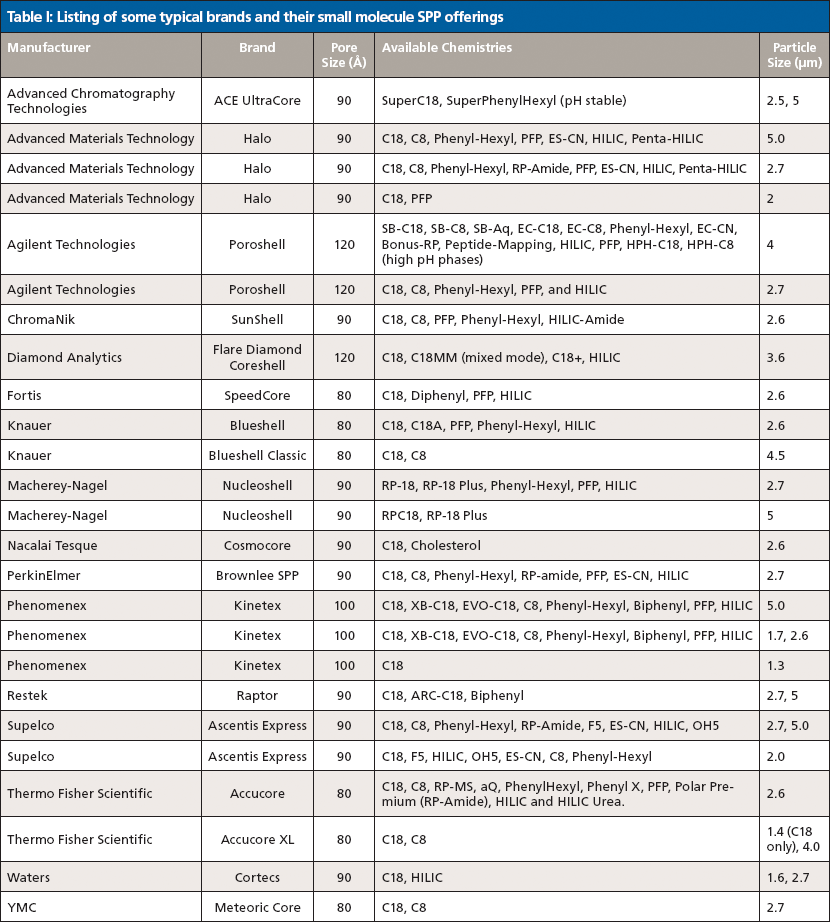
Particles in the range of 2.5 μm appear to be most often used for initial method development because typical back pressures allow them to be utilized on both UHPLC and HPLC systems. The smaller particle sizes tend to be used in high-throughput environments where speed of analysis is important. Larger particle sizes have found use in routine testing laboratories where HPLC systems continue to dominate. It is unclear if even larger or even smaller SPP versions will find utility, but it is expected that further developments within the current range of 1.3–5 μm particle sizes will continue.
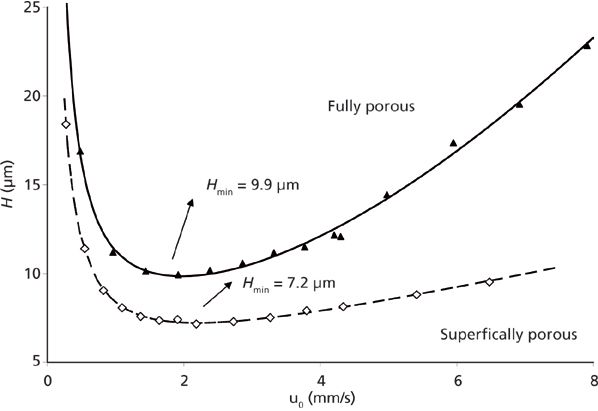
Figure 3: Efficiency comparison between fully porous and superficially porous 5-μm particles. Column dimensions: 250 mm × 4.6 mm, 5 μm; mobile phase: acetonitrile–water; test compound: benzophenone; k' = 6.2 (isoelutropic conditions). (Data courtesy of Dr. Ken Broeckhoven, Vrije Universiteit Brussel.)
Additional Chemical Surface Modifications
It is also apparent from Table I that a wide variety of surface chemistries are now available that use the SPP technology. Because resolution is dependent on efficiency and selectivity, both the particle design (efficiency) and the surface chemistry (selectivity) are significant toward achieving separation (9). Classical surface chemistries such as C18-, C8-, and phenyl-modified surfaces have been commercially available since the inception of SPPs. More unique surface chemistries, such as polar phases suitable for hydrophilic-interaction chromatography (HILIC) (10) and biphenyl phases continue to be commercialized that broaden selectivity options. Several presentations demonstrating that this trend is continuing were presented at Pittcon 2015. For example, Dan Armstrong's group from the University of Texas at Arlington reported the development of several HILIC phases bonded to SPP surfaces (11). The same group also demonstrated improved efficiency for chiral separations when chiral selectors are applied to the SPP technology (12,13). Figure 4 shows a comparison between cyclofructan-based chiral stationary phases (CSPs) bonded to totally porous and superficially porous particles. The improved efficiency of SPP technology results in increased resolution. The group also demonstrated very fast chiral separations using a number of different brush-type chiral stationary phases (CSPs) bonded to shell particles. Other examples include separate reports from Phenomenex and Agilent representatives on the development of high-pH-stable SPP-based phases (14,15). The broadening of selectivity on SPP architecture through additional surface chemistries is expected to continue in the foreseeable future.
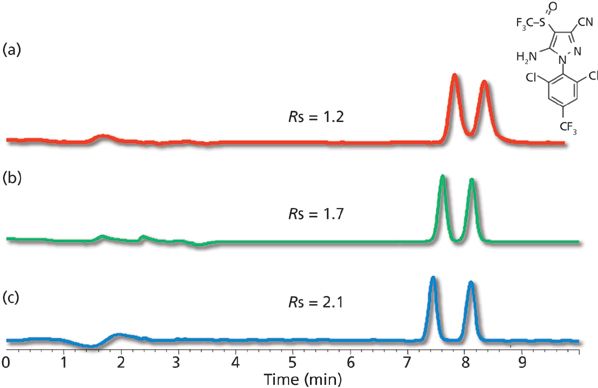
Figure 4: Comparison of totally porous particles and superficially porous particles in chiral chromatography. Columns: Cyclofructan 6 (CF6-P) bonded to (a) 5-μm fully porous particles, (b) 3-μm fully porous particles, and (c) 2.7-μm superficially porous particles; column dimensions: 150 mm × 4.6 mm; mobile phase: heptane–ethanol, 92:8 for the fully porous particles and 95:5 for the superficially porous particles; analyte: fipronil. (Adapted with permission from reference 13.)
Application Beyond Small-Molecule Reversed-Phase Analyses
Another recent trend has been the adaptation of SPP technology for large-molecule separations. The initial SPP offerings were geared toward small-molecule separations with particle pore sizes around 100 Å. Table II lists manufacturers and selected stationary-phase characteristics of recently developed larger-pore SPP columns. According to Fekete and colleagues (16), large-molecule separations suffer from potential issues because of adsorption, secondary interactions, low diffusion coefficients, and poor kinetics. The same features of SPP phases that have proven effective in small-molecule separations are also applicable to large-molecule separations. To allow unrestricted access into the pores for these large molecules, wider-pore structures are required. According to Schuster and colleagues (21), 90 Å pore structures allow unrestricted access to the internal volume for compounds up to about 5 kDa. Particles designed with 160-Å pores provide access to compounds up to about 15 kDa, and those with 400-Å pores provide access for structures up to around 500 kDa. Particles with pore sizes around 160 Å are generally used for peptides and small proteins, whereas larger proteins require further increases in average pore size (17).
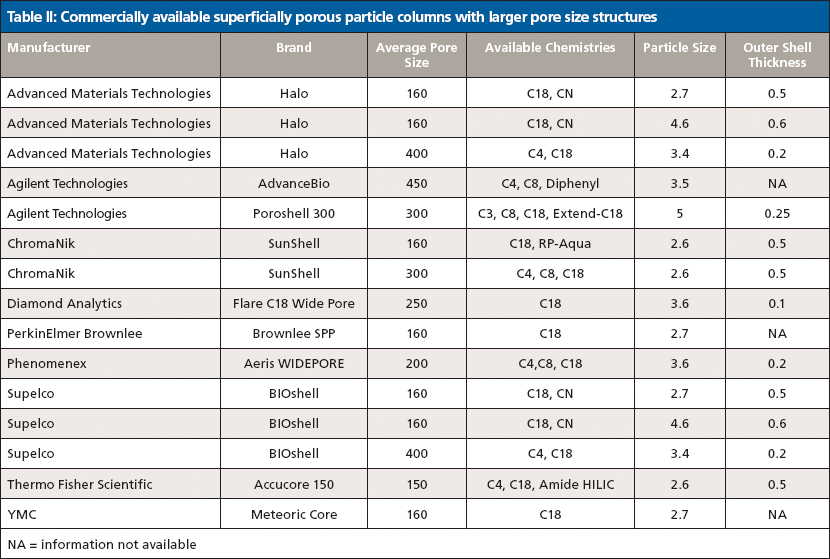
One characteristic difference between the newly developed wide pore SPP packings and the initial small-molecule particles lies in the shell thickness. The porous layer used in particles developed for large-molecule separations are generally thinner than SPPs first introduced for small molecule separations. This is because of the relatively slow diffusion of large molecules in and out of the porous layer. Schuster and colleagues (18) compared several shell thickness layers for efficiency in protein separations. The thinner the porous layer, the more the efficiency increased; however, thinner layers also result in lower loading capacity, thus some compromise is typically arrived at (19). Demonstrations of effective separations of intact proteins and monoclonal antibodies (mAbs) along with performance evaluations have been published recently (18,20–22). Figure 5 shows a comparison of a wide-pore sub-2-μm fully porous column and a 3.6-μm wide-pore SPP phase for the analysis of an intact monoclonal antibody, rituximab. It is apparent from these traces that both particle technologies provide excellent peaks shape and selectivity for this 150-kDa antibody, although in this case the FPP provides slightly better resolution. The same wide-pore SPP phase is used to separate fragments of a digested mAb, bevacizimab, in Figure 6. The efficiency of the SPP particle results in excellent separation of fragments in both the Fc and Fab regions.
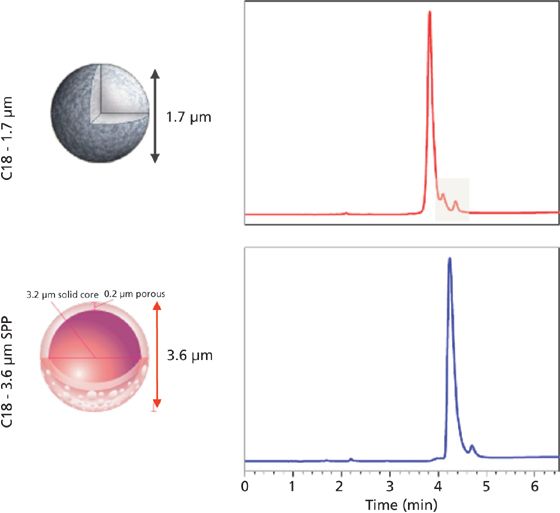
Figure 5: Comparative analysis of intact mAb rituximab (IgG1) using sub-2-μm totally porous and 3.6-μm superficially porous phases. Column dimensions: 150 mm × 2.1 mm; temperature: 80 °C (C18, 1.7-μm, 300-Å) and 90 °C (C18, 3.6-μm SPP, wide-pore). (Data courtesy of Davy Guillarme, University of Geneva.)
Activity around large-molecule SPP developments was also apparent at the recent Pittcon meeting. J.J. Kirkland, in commemorating his receipt of the LCGC Lifetime Achievement in Chromatography Award, presented a talk entitled "Tools to Improve Protein Separations" that focused on recent advances in SPP technology from Advanced Materials Technologies (23). As with small-molecule SPP packings, it is expected that continuous developments in surface chemistry aimed at improving large-molecule separations will continue to emerge.
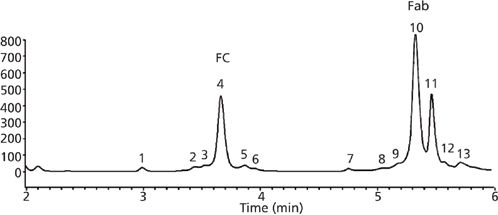
Figure 6: Separation of digested mAb (bevacizumab Fc and Fab fragments). Column: 150 mm × 2.1 mm, 3.6-μm widepore SPP, C18; mobile-phase A: 0.1% trifluoroacetic acid in water; mobile-phase B: 0.1% trifluoroacetic acid in acetonitrile; gradient: 30–40% B over 11 min; flow rate: 0.35 mL/min; temperature: 90 °C; injected volume: 0.5 μL; detection: fluorescence (excitation at 280 nm, emission at 360 nm). (Data courtesy of Davy Guillarme, University of Geneva.)
In addition to expansion of SPP technology to large-molecule reversed-phase separations, exploration of SPPs for use in ion chromatography and supercritical fluid chromatography (SFC) have surfaced. For instance, at the Emerging Separations Technologies Symposium organized by The Chromatographic Society and the Separation Science Group of the Analytical Division of the Royal Society of Chemistry held on March 26, 2015, in London, UK, Davy Guillarme presented compelling evidence that SPP particles provide enhanced efficiency over traditional particles in SFC (24). Figure 7 shows a comparison of FPP and SPP phases based on the van Deemter relationship in SFC. As with HPLC and UHPLC, SPP technology affords increased efficiency over similarly sized particles in SFC. The alternative selectivity, as demonstrated in Figure 8 using SPP technology bonded with different polar phases, indicates that the trend of broadening selectivity through modifications of surface chemistry is spreading to the alternative techniques as well. Several recent publications support SPP use in SFC (25–28).
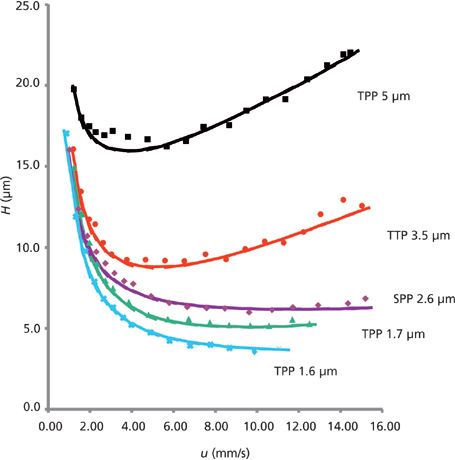
Figure 7: Comparison of superficially porous and totally porous particle performance in SFC. Mobile phase: 98:2 carbon dioxide–methanol; model compound: butylparaben. (Data courtesy of Davy Guillarme, University of Geneva.)
Conclusion
HPLC columns packed with SPPs are rapidly becoming a preferred tool for separations method development in the modern analytical laboratory. SPPs continue to expand in their traditional realm of small-molecule separations through the introduction of alternative surface chemistries and a widened assortment of particle sizes. SPPs with wider pore structures have recently been made available that allow large molecules such as proteins and large peptides to be effectively separated. The particle technology also continues to expand into additional separation techniques such as SFC. Further developments in surface chemistry modifications and fundamental investigations into particle architecture promise even further extension of the SPP platform utility.
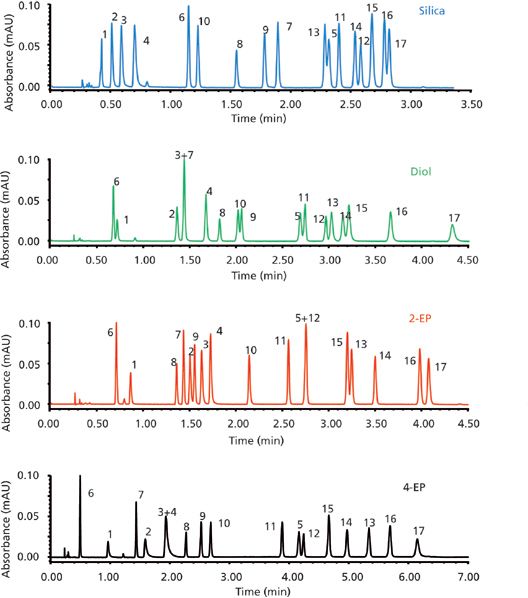
Figure 8: Alternative selectivity for acidic and neutral compounds using various polar SPP columns in SFC. Silica column (Sunshell): 5–20% methanol in 4 min; diol column (Sunshell), 2-EP column and 4-EP column: 5–25% methanol in 5.33 min; flow rate: 2.5 mL/min; back pressure: 150 bar; temperature: 40 °C; injected volume: 1 μL; detection: UV absorbance at 220 nm. Peaks: 1 = ibuprofen, 2 = flurbiprofen, 3 = naproxen, 4 = ketoprofen, 5 = paracetamol, 6 = caffeine, 7 = etophylline, 8 = thymine, 9 = uracil, 10 = warfarin, 11 = hydrocortisone, 12 = prednisolone, 13 = sulfamethoxazole, 14 = sulfadimethoxine, 15 = sulfamethazine, 16 = sulfaquinoxaline, 17 = sulfamethizole. (Adapted with permission from reference 23.)
Acknowledgments
The author would like to acknowledge the following reviewers for their substantial suggestions to improve this work. Dr. Davy Guillarme of the University of Geneva, Dr. Zachary Breitbach of the University of Texas at Arlington, and Dr. Ron Majors of LCGC.
References
(1) V. González-Ruiz, A.I. Olives, and M.A. Martín, Trends Anal. Chem.64, 17–28 (2015).
(2) S. Fekete, E. Oláh, and J. Fekete, J. Chromatogr. A1228, 57–71 (2012).
(3) F. Gritti and G. Guiochon, J. Chromatogr. A1228, 2–19 (2012).
(4) S. Fekete and D. Guillarme, LCGC North Am.32(6), 420–433 (2014).
(5) G. Guiochon and F. Gritti, J. Chromatogr. A1218, 1915–1938 (2011).
(6) S. Deridder and G. Desmet, J. Chromatogr. A1218, 46–56 (2011).
(7) K. Broeckhoven, D. Cabooter, and G. Desmet, J. Pharm. Anal.3, 313–323 (2013).
(8) G. Kahsay, K. Broeckhoven, E. Adams, G. Desmet, and D. Cabooter, Talanta122, 122–129 (2014).
(9) C.R. Aurand, H. Cramer, J. McKenzie, and D.S. Bell, LCGC North Am.32(9), 704–717 (2014).
(10) D.S. Bell, LCGC North Am.33(2), 90–101 (2015).
(11) M.D. Dolzan, D.A. Spudeit, Z.S. Breitbach, W.E. Barber, G.A. Micke, and D.W. Armstrong, J. Chromatogr. A1365, 124–130 (2014).
(12) D.A. Spudeit, M.D. Dolzan, Z.S. Breitbach, W.E. Barber, G.A. Micke, and D.W. Armstrong, J. Chromatogr. A1363, 89–95 (2014).
(13) D.C. Patel, Z.S. Breitbach, M.F. Wahab, C.L. Barhate, and D.W. Armstrong, Anal. Chem. in press (2015).
(14) L.Y. Loo, L. Abadilla, M. Chitty, I. Rustamov, T. Tran, and T. Farkas, "The Benefits of an Optimized and Robust High pH Stable Core-Shell Stationary Phase for the Analysis and Purification of Basic Analytes," Presented at Pittcon 2015, New Orleans, Louisiana, 2015.
(15) W. Long, A.E. Mack, J. Link, and X. Wang, "Examining Orthogonal Separations in Superficially Porous Particles: Maximizing Resolution Through the Use of Bonding Chemistries and New High pH Stable Columns," Presented at Pittcon 2015, New Orleans, Louisiana, 2015.
(16) S. Fekete, J. Schappler, J.-L. Veuthey, and D. Guillarme, Trends Anal. Chem.63, 2–13 (2014).
(17) D.S. Bell, H.K. Brandes, and D. Wallworth, in Chromatography Today8(1), 4–8 (2015).
(18) S.A. Schuster, B.M. Wagner, B.E. Boyes, and J.J. Kirkland, J. Chromatogr. A1315, 118–126 (2013).
(19) S. Fekete, J.L. Veuthey, and D. Guillarme, J. Pharm. Biomed. Anal. 69, 9–27 (2012).
(20) S. Fekete, R. Berky, J. Fekete, J.L. Veuthey, and D. Guillarme, J. Chromatogr. A1236, 177–188 (2012).
(21) S. Fekete, S. Rudaz, J. Fekete, and D. Guillarme, J. Pharm. Biomed. Anal.70, 158–168 (2012).
(22) B.M. Wagner, S.A. Schuster, B.E. Boyes, and J.J. Kirkland, J. Chromatogr. A1264, 22–30 (2012).
(23) J.J. Kirkland, S.A. Schuster, B.M. Wagner, and B.E.Boyes, "Tools to Improve Protein Separations," Presented at Pittcon 2015, New Orleans, Louisiana, 2015.
(24) D. Guillarme, "The use of modern supercritical fluid chromatography in pharmaceutical analysis," Presented at Emerging Separations Technologies, RSC Burlington House, Piccadilly London, UK, 2015.
(25) A.G.G. Perrenoud, W.P. Farrell, C.M. Aurigemma, N.C. Aurigemma, S. Fekete, and D. Guillarme, J. Chromatogr. A1360, 275–287 (2014).
(26) S. Delahaye, K. Broeckhoven, G. Desmet, and F. Lynen, Talanta116, 1105–1112 (2013).
(27) A. Grand-Guillaume Perrenoud, J.-L. Veuthey, and D. Guillarme, Trends in Anal. Chem. 63, 44–54 (2014).
(28) E. Lesellier, J. Chromatogr. A1228, 89–98 (2012).
This month's guest coauthor:
David S. Bell is a manager in pharmaceutical and bioanalytical research at Sigma-Aldrich/Supelco. With a B.S. degree from SUNY Plattsburgh and a PhD in Analytical Chemistry from The Pennsylvania State University, Dave spent the first decade of his career within the pharmaceutical industry performing analytical method development using various forms of chromatography and electrophoresis. During the past 15 years, working directly in the chromatography industry, Dave has focused his efforts on the design, development, and application of stationary phases for use in HPLC and hyphenated techniques. In his current role at Supelco, Dr. Bell's main focus has been to research, publish, and present on the topic of molecular interactions that contribute to retention and selectivity in an array of chromatographic processes. Direct correspondence to: dave.bell@sial.com

David S. Bell
Editor of Column Watch:
Ronald E. Majors is the editor of "Column Watch," an analytical consultant, and a member of LCGC's editorial advisory board. Direct correspondence about this column to lcgcedit@lcgcmag.com

Ronald E. Majors

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).















