The Secrets of Successful Gradient Elution
LCGC North America
What is required to optimize a gradient elution HPLC method?
What is involved in developing and optimizing a gradient elution reversed-phase high performance liquid chromatography (HPLC) method? Several factors need to be considered.
Blank Gradient
First, run a blank gradient to ensure there are no problems with baseline drift. Drifting baselines are an insidious problem in gradient elution and are caused by the change in eluent composition. Changes in UV absorbance and refractive index properties are detected, which results in a rising or falling baseline. If possible, change the wavelength that is being monitored (especially at low wavelengths) or use a solvent that does not absorb at the wavelength being monitored. If a diode-array detector is being used, a reference wavelength can be set that compensates for changes in the absorbance and refractive index of the background.
Scouting Gradient
Run a scouting gradient of 5–100% B over 20 min using a mobile-phase A of 10 mM ammonium formate, pH 2.8; a mobile-phase B of acetonitrile; a 150 mm x 4.6 mm, 5-µm C18 column; and a flow rate of 2 mL/min. The use of volatile ammonium formate as a buffer provides the added advantage of making the method compatible with mass spectrometry detection (or other detection modes that require volatile buffers). The chromatographic results allow gradient parameters to be optimized (1). Start by optimizing the initial and final %B based on the retention times of the first and last eluted peaks (equations 1–3) (1).
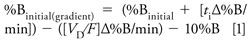
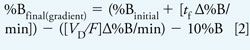
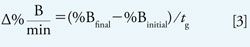
In equations 1–3, %Binitial = initial %B used in scouting gradient, %Bfinal = final %B used in scouting gradient, tg = gradient time, ti = elution time initial peak (min), tf = elution time final peak (min), VD = dwell volume (mL), and F = flow rate (mL/min).
Gradient Steepness
Optimize gradient steepness using the conditions found from the scouting gradient (equation 4):
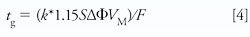
In equation 4, k* = gradient retention factor (use 5 as starting point), S = shape selectivity factor (for small molecules use 5 or calculate, S = 0.25MW0.25), and Δφ = change in %B expressed as a decimal, and VM = column interstitial volume (mL). Gradient slope can alter resolution and selectivity; as the slope decreases, resolution increases due to a change in k*.
Reequilibration Time
The next step is to optimize reequilibration time (equation 5) (2):
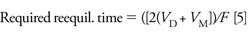
It is an important step, and if it is not properly considered it can lead to retention time and quantitative variability.
Fine-Tune Parameters
The gradient time can be varied to assess the effect on the analysis (vary by twofold or more); changes in resolution of critical pairs should be noted. When varying the gradient time, the initial and final %B values may need to be adjusted. If further optimization is required, vary the solvent type (neutral samples), followed by column chemistry. For ionizable analytes, variations in pH or temperature should be investigated before changing column chemistry. Gradient steepness should be reoptimized following any changes in solvent or column.
Analysis Time
After conditions have been optimized using the steps above, the analysis time can be reduced by varying the flow rate, column length, or particle size. If the flow rate has been changed, a final adjustment of the reequilibration time can be made to optimize overall analysis time. When adjusting column conditions, selectivity, but not necessarily resolution, can be kept constant (that is, the same elution order) by maintaining k* using the gradient steepness (GS). Change the flow rate: alter tG to keep GS constant; change the column length: alter tG or F to keep GS constant; change the column internal diameter: alter tG or F to keep GS constant (equation 6).

Dwell Volume
Dwell volume differences between systems may cause changes in selectivity and resolution when transferring methods (3). The solution is to insert an isocratic hold or use injection delay. System wash-out volume will also affect method transfer. This is the time taken for the instrument to wash-out the strong solvent channel and can be as much or more than the dwell volume. This can affect reequilibration, which cannot occur until the initial eluent conditions are reached.
References
- http://www.chromacademy.com/chromatography-Optimizing-Gradient-HPLC-Parameters.html
- A.P. Schellinger, D.R. Stoll, and P.W. Carr, J. Chromatogr. A1064, 143–156 (2005).
- The Essentials: Troubleshooting Real HPLC Problems, The Column12, 18–19 (2014).
- Find this webcasts at http://www.chromacademy.com/Gradient-HPLC.html?tpm=1_1 (free until June 20).

Determining the Link Between Prenatal Cannabis Use and Symptoms of Depression Using LC–MS/MS
April 16th 2025Researchers investigating the relationship between cannabis use during pregnancy and depressive symptoms—and whether continued use beyond the first trimester or higher levels of use were linked to increased symptoms—used liquid chromatography–tandem mass spectrometry (LC–MS/MS) to confirm the presence of 11-nor-9-carboxy-delta-9-tetrahydrocannabinol (THC-COOH) in urine samples.
A Guide to (U)HPLC Column Selection for Protein Analysis
April 16th 2025Analytical scientists are faced with the task of finding the right column from an almost unmanageable range of products. This paper focuses on columns that enable protein analysis under native conditions through size exclusion, hydrophobic interaction, and ion exchange chromatography. It will highlight the different column characteristics—pore size, particle size, base matrices, column dimensions, ligands—and which questions will help decide which columns to use.














