Revival of Capillary Electrophoretic Techniques in the Pharmaceutical Industry
LCGC North America
A look at what influences the use of CE in the pharma industry.
The first observations of electrophoresis were made in 1807 by Ferdinand Frederic Reuss. Arne Tiselius (1902–1971) introduced electrophoresis as an analytical technique in 1937 (1) and was rewarded with a Nobel Prize for it in 1948 (2). One of his PhD students was Stellan Hjertén (1928–), who further developed the technique in open tube electrophoresis. His thesis was published in English in 1967 in Chromatographic Reviews (3) and he has been rewarded many times over the years for his pioneering work, most recently he was given the 2011 CASSS Award for Separation Science (4). Other pioneers in the field include Virtanen (5) and Mikkers and Everaerts (6) who performed electrophoresis in 200-μm PTFE and glass tubing. In 1981, Jorgenson and Lukacs made the important step to apply 75-μm fused-silica capillaries (7); afterward capillary electrophoresis (CE) really took off.
What Is Capillary Electrophoresis?
CE can be described as an automated, analytical version of the conventional electrophoresis techniques. The two sidebars in this article explain the separation principles of CE. The main advantages of performing electrophoresis in a capillary are the magnificent efficiencies and the automation possibilities. Because of the small diameters of the capillary, typically in the 20–100 μm inner diameter range, the Joule heat dissipation is very efficient. Consequently, high voltages, usually up to 30,000 V, can be applied before excessive Joule heating becomes an issue. Applying high voltages results in fast separations with very little band broadening.
Usually, the capillary is made from fused silica, so on-column UV detection is feasible. Therefore, time-consuming staining and destaining procedures as seen with slab-gel electrophoresis are no longer needed. The combination of the small-inner-diameter capillary and on-column UV detection means that automated equipment has been developed with peaks resulting in electropherograms, simplifying quantitative analysis. Small bands show as efficient peaks with high plate numbers.
Electrophoresis separates analytes that differ in charge-to-size ratio, so charge is a prerequisite for separation by CE. Several solutions have been created to induce charge and size differences in a capillary, as well as to add extra separation mechanisms. Table I lists some of the most frequently used modes of CE.

Table I: The different modes of capillary electrophoresis
CE is applicable over a wide range of analytes. Anything from small anions and cations to chiral separations, large proteins, DNA, cell organelles, and even complete cells and viruses have been analyzed with CE. The small scale of CE makes it a very green technique. Typically, a 50-cm long, 50-μm i.d. capillary has a capillary volume of 1 μL. Most often the separation medium is aqueous. So, the consumption and cost of chemicals are very low.
The small scale of the techniques also means that only a few nanoliters of sample are injected. This is advantageous for application areas in which sample size is an issue, such as bioanalysis or the discovery phase of drug development.
Orthogonality
Electrophoresis is a fundamentally different separation technique from chromatography. Chromatographic separations are based on partition differences of the analytes between the stationary and mobile phase. Electrophoresis is based on differences in migration of charged particles in an electric field. Consequently, chromatography and electrophoresis are complementary tools in the analytical chemist's toolbox. Of course, there are separation problems that can be solved with both techniques. But, even so, there are problems for which one technique proves superior. The art of good analytical science is to select the tool that uses the strength and thus robustness of a technique rather than apply it at the limit of its capabilities and, therefore, have it be intrinsically less robust.
Practical Aspects of the CE Capillary
Initially, fused silica was used as a capillary column because of its inert nature, UV transparency, and low cost. However, "inert" is not a very apt word in this case. The use of fused silica for CE results in the so-called electro(end)osmotic flow (EOF, see sidebar). As can be understood from the origin of it, the EOF is sensitive to anything influencing the capillary wall and the protonation or deprotonation of the silanol groups. Thus, for good precision it is important to understand and control the EOF process well. Early on, we understood the importance of caustic rinsing procedures to condition new fused-silica capillaries. Presequence and prerun conditioning procedures have been (and should be) the point of attention for all method developers because these procedures are method specific. Besides conditioning of the wall, there can be other reasons for preconditioning steps:
- refreshing the separation medium to prevent buffer depletion;
- reducing analyte–wall interactions;
- preventing carryover from highly concentrated sample components;
- and flushing out late-migrating components that are of no interest.
Table II lists examples of potential conditioning steps (8). The simplest step, rinsing with the separation buffer, should always be part of the prerun conditioning. Other steps might be added as needed.

Table II: Possible preconditioning steps
Still, a good conditioning scheme is sometimes not sufficient to control the stability, magnitude, and direction of the EOF and cannot always sufficiently suppress analyte-wall interactions. In light of this, coating procedures have become more and more common, especially adsorbed coatings. These can be divided into two groups, static adsorbed and dynamic coatings. Examples of the static adsorbed coatings are polymer coatings such as the cationic polybrene and the anionic dextran sulfate and poly(vinylsulfonic acid). These coatings are very stable and effective as double- and triple-layer coatings, also called successive multiple ionic polymer layer (SMIL) coatings (see reference 9 for a review). If properly applied, these coatings are stable for many runs and the coating polymer does not need to be present in the background electrolytes (BGE). This gives more flexibility to optimize the BGE independent of the coating. SMIL coatings are being applied in many commercial kits, such as CEofix (Analis), and also for many CE–mass spectrometry (MS) analyses of proteins.
The other group of adsorbed coatings are the so called dynamic coatings. These have to be present in the BGE for an effective, repetitive, and reproducible effect. Dynamic coatings can be ionic surfactants such as cetyl trimethylammonium bromide (CTAB), monoamines such as triethanolamine, diamines such as putrescine, or polyamines such as spermine. To demonstrate how well-used these coatings are, here are two examples from the pharmacopeias: Triethanolamine is being used in the pharmacopeial method for the enantiomeric purity of S-ropivacaine (Figure 1 [10,11]) and putrescine is being used in the pharmacopeial method for the separation of erythropoietin (EPO) isoforms.

Figure 1: The enantiomeric purity determination of (S)-ropivacaine. Capillary: 80.5 (72.0) cm à 50 μm fused silica; background electrolyte (BGE): 100 mM phosphoric acid, 88 mM triethanolamine, and 10 mM DM-β-CD, freshly prepared and filtered over 0.45-μm pore size PTFE filters; BGE pH: 3.0; applied voltage: 30 kV (current 42 μA) with initial ramping of 500 V/s; temperature: 30 °C; injection: 50 mbar à 5 s; detection: UV absorbance at 206 nm with 4 nm bandwidth; prerun conditioning: 1 min flush of water, 4 min of 0.1 M sodium hydroxide, 1 min of water, and 4 min of BGE (before the start of a sequence the capillary is rinsed for 10 min with 0.1 M sodium hydroxide or for 30 min for a new capillary); capillary storage: stored dry after a rinse for 10 min with sodium hydroxide, 10 min with water, and 10 min from empty vials; sample: 2 mg/mL ropivacaine hydrochloride solution for injection, directly injected without further sample preparation. Quantification by internal normalization of corrected peak areas. The %RSD for the amount of (R)-ropivacaine in this sample was 8%. Sample preparation of other ropivacaine products: higher concentrations of solution for injection are diluted with water to 2 mg/mL. Ropivacine HCl substance is dissolved in water to 2 mg/mL.
As discussed, anything influencing the capillary wall can affect precision and reproducibility. Therefore, it is also important to realize that a capillary has a history and to stick with one application per capillary. Furthermore, after method development the capillary has probably "seen" separation buffer components or rinsing solutions that did not make it to the final method. So after method development, it is a good practice to recheck the final optimized conditions on a fresh capillary.
There are other details, such as the cutting of the capillary, that are part of good CE working practices that are important to get sharp, reproducible peaks. Although not difficult (12) and easy to check, improper cutting often gives rise to loss of efficiency and resolution (see Figure 2 ). Even a broken outlet might result in sloping baselines or tailing peaks. Remove the outer polyimide coating a few millimeters from the inlet and outlet of the capillary. This will reduce carryover and give better precision. This is important especially when using solvents that make the polyimide swell, such as acetonitrile. Removing the polyimide from the inlet and outlet is not advisable for some coated capillaries because it might damage the inner coating.

Figure 2: Sample zone and peak distortion by badly cut or broken capillary: (a) Conditioned capillary filled with BGE; (b) inlet BGE vial replaced by sample vial; (c) injection of sample; (d) inlet BGE vial back in place, migration ongoing; (e) blue: peak shape that results from the distorted zone shape; red: peak shape if the capillary inlet had been straight and the sample zone normal.
Do the Pharmaceutical and Biotech Industries Really Use CE?
So, is CE really being used in industry? An academic friend recently remarked that industry must be using CE less than before since the number of scientific publications from industry is declining. Others have made similar remarks. Other rumors are that CE is not reproducible and robust, so it cannot be used for quality control (QC). Additionally, people think that CE methods cannot be validated, and even that regulatory bodies such as the United States Food and Drug Agency (FDA) do not like CE. Are these comments really supported by the facts?
The facts are that there are many successful methods during all stages of development within industry, although this cannot be fully proven by publications. There are adopted methods in use including applications from the pharmacopeias such as the United States Pharmacopeia (USP) and European Pharmacopoeia (Ph.Eur.) (for a selection of these methods, see Table III; for a review see references 13 and 14). Of course, to get as far as this, these methods have to be successfully validated. Experiences from the field and sometimes reports in peer-reviewed publications show that for CE, similar robustness and reproducibility is possible, as with other techniques and methods such as high performance liquid chromatography (HPLC). There are several successful intercompany studies using multiple modes of CE (15–21). The recent increase in sales of instruments show that CE is still a growing market.
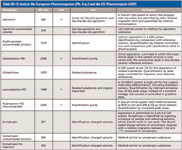
Table III: CE tests in the European Pharmacopoeia (Ph. Eur.) and the US Pharmacopeia (USP)
Furthermore, there is a good knowledge base within the regulatory bodies of CE techniques. Representatives actively participate in symposia and are part of organizing committees such as for CE in the Biotechnology & Pharmaceutical Industries (CEPharm). This CASSS-run meeting has seen a steady attendance from industry, vendors, and regulatory bodies, and had its 14th meeting this year.
If one submits CE methods to regulatory bodies, they receive the same attention as other submitted methods. Just as with other methods, one must justify the need for the method and provide the thorough information needed for reviewing the submission, irrespective of the technology used.
Applications from the Pharmaceutical Industry
CE is being used by the pharmaceutical and biotech industry in all phases of drug development: not only for identity, assay, and purity analysis of the active pharmaceutical ingredient (API), but also for other types of applications such as physical-chemical characterization (pKa, logP, and logD determinations and drug–protein binding), analysis of counterions, formulation development support, conformation of results obtained by other techniques, and troubleshooting. Because of the proprietary nature of the drug substances being investigated in the pharmaceutical industry, many of these applications never get published. Here are some examples that illustrate the use of the different CE modes.
Capillary Zone Electrophoresis
There are many similar methods that are successfully being applied for the determination of drug countercations and counteranions. Mostly, these methods use indirect UV detection. An excellent book chapter on ion analysis was written by De l'Escaille and Falmagne (22). Figure 3 shows an example of the determination of bromide as potential impurity in HCl drug salts with CE and direct UV detection (23). Indirect UV detection has limited loadability and linearity and is less suitable for determining the presence of a large excess of chloride because electromigration dispersion of the highly concentrated chloride peak may impair the resolution. The direct-UV bromide determination was optimized with respect to the resolution of the chloride and the bromide peaks by statistical experimental design using a multivariate optimization program. A sample matrix containing a high concentration of an ion such as chloride may cause antistacking. However, if the mobility of the chloride ion is lower than the mobility of the bromide ion, the high chloride concentration may contribute to a sample self-stacking effect. Furthermore, the buffer co-ion must be carefully chosen with respect to its mobility to achieve an efficient separation system (23).
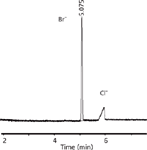
Figure 3: Determination of bromide in hydrochloride drug salts using CE with direct UV detection. Capillary: 50 μm i.d., effective length 41.5 cm, fused silica, extended light path (bubble factor 3); background electrolyte: 60% acetonitrile and 100 mM methanesulfonic acid adjusted to pH 1.3 with triethanolamine; sample: local anesthetic HCl salt containing 0.3% (w/w) bromide; voltage: -15 kV; temperature: 20 °C.
An example from the biotech industry is the charge heterogeneity determination of proteins such as monoclonal antibodies. Yan He of Pfizer (24) published a rapid analysis on short, dynamically coated fused-silica capillary. The pH, concentration of the separation buffer (?-amino caproic acid), concentration of the triethylenetetramine (TETA) dynamic coating, the capillary internal diameter, and the applied field strength were investigated and optimized. He also evaluated the effects of between-run flushing of the capillary and the data acquisition rate. Compared with other existing methods for charge variants analysis, this method had several advantages including a short run time, rapid capillary conditioning and simple sample preparation. At the MicroScale Bioseparations (MSB 2012) meeting in Geneva, Switzerland, Bernd Moritz of Roche showed that the same methodology on a longer capillary results in improved resolution of the isoforms, at the cost of longer analysis times (25).
The pharmacopeial test method for identity and assay of erythropoietin (EPO) is also a capillary zone electrophoresis (CZE) method with a dynamically coated capillary, in this case with putrescine (1,4-butanediamine). Long rinsing procedures are required, but when optimally conditioned, the method shows highly resolved and efficient peaks for the EPO isoforms (26). In the pharmacopeial identification and charge variants test method for somatropin, somatropin concentrated solution, and somatropin for injection, deamidated charged variants migrate after the main peak (27).
Micellar Electrokinetic Chromatography and Microemulsion Electrokinetic Chromatography
In micellar electrokinetic chromatography (MEKC), micelles are added to the separation solution to separate uncharged analytes. Uncharged analytes partition between the more lipophilic inner part of the micelles and the hydrophilic aqueous buffer. So part of the time they move with the micelles, and part of the time with the EOF. Their migration order will depend on the strength of the interaction with the micelles: the more lipohilic the analyte, the stronger the interaction. That means that uncharged analytes will show an effective mobility that is between the mobility of the micelles and the mobility of the EOF. Charged analytes will experience both this chromatographic effect and the electrophoretic forces, and their net mobility is harder to predict.
An elegant example of a MEKC method was published by K. Persson-Stubberud (28,29). She developed, validated, and submitted for regulatory approval one method for the quantification of ibuprofen and codeine and the determination of degradation products in commercial tablets. Factorial design was used for both method development as well as robustness testing.
If the analytes of interest are more lipophilic, using microemulsions in microemulsion electrokinetic chromatography (MEEKC) might be a better solution than using micelles as additives for optimal separation. An interesting approach in problem solving using MEEKC was nicely demonstrated by McEvoy (30). Suppositories containing paracetamol were dissolved and analyzed with the same microemulsion, making sample preparation simpler.
Chiral CE
CE is a strong tool for chiral separations since the chiral selector is dissolved in the separation buffer. If the dynamic complexation with the chiral selector differs between the enantiomers or if their complexes differ in mobility, they can be separated. Because the chiral selector is in the separation solution, a whole series of chiral selectors can be screened in a short time. On top of that, the amount of chiral selector and its concentration in the buffer can vary. Variation of the chiral selector concentration is an important parameter in method optimization.
Although one can fairly successfully predict interaction of a compound with a selector, it is still virtually impossible to predict enantiomeric separation; for example, see Figure 4 and references 31 and 32. Consequently, it is advisable to test as many selectors as possible in a screening experiment. The most frequently used chiral selectors are cyclodextrins, as they are UV-transparent and a wide variety of substituted cyclodextrins are commercially available. Figure 4 shows the enantiomeric purity separation of S-ropivacaine, a method that has been taken up in the pharmacopeias. Under exactly the same conditions (BGE: 100 mM phosphoric acid, 90 mM triethanolamine, 10 mM DM-β-CD), some of the enantiomer pairs are very well separated (for example, resolution of 7.5 between DLDD and LDLL), and some are not separated at all, such as DLDL and LDLD. All chiral forms of the tetrapeptide showed association complexes with the cyclodextrin, as was observed by migration time shifts for all after adding the cyclodextrin to the buffer. The main difference was the association differences for the enantiomer pairs (23).
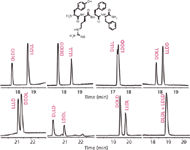
Figure 4: The separation of the 16 stereoisomers of the tetrapeptide Tyr-Arg-Phe-Phe-NH2, presented as eight enantiomer pairs.
Isoelectric Focusing
Within the biotech industry, conventional isoelectric focusing (IEF) has been replaced by capillary isoelectric focusing (cIEF) in many cases. cIEF is either performed on a short coated capillary with whole-column detection, on a so-called imaging instrument (icIEF), or on a traditional CE instrument. In both cases, the sample is mixed with ampholyte and the capillary is filled with this mixture. When the voltage is applied, the ampholyte forms a pH gradient through the capillary and the proteins migrate in this pH gradient until they reach the pH where their net charge is zero — that is, where the pH equals the isoelectric point (pI). The main advantage of performing IEF in a capillary is its speed and quantitative capabilities. There are generic methods and standard kits available. Traditional ampholytes are being used, although some have better UV properties than others. The major parameters for method optimization are in the sample prep procedure, that is, desalting and the amount of urea needed.
Yan He compared cIEF with CZE for monoclonal IgG (24) and found that overall, icIEF showed better separation of acidic isoforms, whereas CZE showed better separation of basic isoforms.
Capillary Gel Electrophoresis
In capillary gel electrophoresis (CGE), a noncrosslinked linear polymer is used to form the sieving gel. Again, the instrument automation and improved quantitation properties make the capillary format interesting. A huge application area is the analysis of DNA, although the capillary form of sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) for proteins receives more attention in the pharma industry. Commercially, the term CE-SDS also is being used for the capillary format of SDS-PAGE. There are commercial kits available for molecular weight determinations of proteins and for the charge heterogeneity determination of monoclonal antibodies (19). With UV detection, Coomassie blue sensitivity is achieved, and with laser-induced fluorescence (LIF) detection silver staining sensitivity is possible. All of the CGE applications for DNA, RNA, and proteins are also available on chip-sized analyzers.

Capillary Electrophoresis
Capillary Electrochromatography
In the early days of capillary electrochromatography (CEC), there was also a distinct interest within pharma for the potentials of this technique. CEC combines a chromatographic partition mechanism with a flat electroosmotic flow profile. A capillary is packed with LC column material and the flow is created by applying a high electric field. For uncharged compounds, the separation mechanism is purely chromatographic, although differences in migration order by micro-LC and CEC on the same column have been observed (33). For charged compounds, the separation is the result of both chromatographic and electrophoretic interactions. An example from industry using chiral CEC is found in reference 34. Currently, to my knowledge, interest in CEC in industry has decreased, partly because of the issues with reproducibility of the capillaries (packing and frit making), and partly because of the introduction of ultrahigh-pressure liquid chromatography (UHPLC). It would be interesting to see if the reduced particle size from UHPLC and the increasing knowledge about packing reproducible capillaries revives interest in CEC.
CE–MS
Although it is hard to get an overview because much remains unpublished, CE–MS is being used sparsely within the pharma industry and when it is used, it is mainly for characterization. In early drug discovery, CE–MS can be used for the simultaneous determination of pKa values of very small amounts of drug mixtures (35,36). Also, for impurity profiling purposes CE–MS is interesting as an orthogonal technique to LC–MS. Visky and colleagues used CE–electrospray (ESI)-MS-MS for the impurity profiling of galantamine hydrobromide in stressed extended release capsules (37). Recently, Whitmore published the tryptic mapping of a protein digest with CE–MS. Tryptic peptide mapping is routinely used in the biotech industry to confirm primary sequence, to determine cell line stability, and to analyze post-translational modifications. With CE–MS, complementary information was obtained compared to LC–MS (38). The peptides found with CE–MS and not with LC–MS contained three amino acids or fewer and included two peptides in the critical complementary binding domain. At CEPharm 2011, there was a special session dedicated to CE–MS that also included a workshop. The high attendance demonstrated an increasing interest from industry to expand the characterization of (protein) pharmaceuticals with CE–MS.

Electrophoresis in a Capillary: The Electroosmotic Flow
Renewed Interest from Pharma
From discussions with users from the pharma industry, it seems that the use of CE declined in the first decade of this century, especially for small molecules. The reasons for this varied. Specialists moved on to managerial functions or disappeared in all the reorganizations. Equipment improvement halted for some time, and application kit development was slow, with the kits developed mainly for protein pharmaceuticals. It also took time for industry to investigate this new technique sufficiently to come up with good working practices. In many cases, the expectations were high and the results were, therefore, disappointing. Many people treated CE the same as LC, but these techniques are fundamentally different, so good working practices from the one are not applicable to the other. Sensitivity and precision suffered as a result. The smaller capillary diameter in CE (compared to the HPLC detection cell) makes for a smaller detection pathlength, although higher efficiencies intrinsically give higher concentrations in the sample zone. With the development and increased knowledge of appropriate injection and stacking techniques for CE, sensitivity similar to that of LC methods has been achievable.
Currently, the revival of CE in the pharma industry seems to come from the biotech area. Here, CE does not "compete" with HPLC or UHPLC, but is an automated and quantitative alternative to traditional gel electrophoresis. Application kits have improved over the years, thanks to many efforts and input from industry, and CGE and cIEF seem well-established in the bigger biotech companies. Companies that want to produce biosimilars find CE methods in the pharmacopeias and need to consider the use of CE as well. Also, smaller innovator companies are becoming more and more interested in the now well-established cIEF and CGE methodology. From experience with the "translated from gel electrophoresis" methods, there is now also an expansion toward other CE modes, especially CZE, and this is visible in the biotech industry.
Keys for Success
The presence of well-developed kits and improved equipment does greatly assist companies in getting started with CE. Companies that start with CE find that it is not sufficient to buy an instrument and application kits and that they need to invest in training and good contact with industrial experts. Over the years, the CE user community has learned a lot about good CE working practices, and it is this knowledge that needs to be spread further for proper implementation of the technique. Combined with good practice, knowledge about injection and enrichment techniques are the keys.
Another driver for the revival of CE in industry is the increasing application of CE–MS. The sheath-flow interface has been on the market now for some time and, although still in the hands of specialists, is increasingly robust and easier to handle. The announcement of the launch of a new commercial sheathless interface that seems more sensitive is welcomed. From several areas, there is evidence that CE–MS gives results that are complementary to LC–MS, and therefore it is a tool not to be missed for characterization and profiling.
Key Risk Factors
More compendial methods will increase the use and implementation of CE methodologies in the pharma industry. For compendial methods, proprietary consumables are not acceptable. For instance, CE-SDS and (i)cIEF methodology are firmly implemented in biotech and pharma, yet we still see an increasing market. However, these methodologies contain proprietary consumables (such as capillary coatings and separation medium compositions), which hinders their becoming compendial, because compendial methods require general availability and independence from equipment brand. Unfortunately, this is not the current situation. Equipment requirements for the successful application of kits is highly appropriate and justified, if properly documented. For both equipment and applications we need more suppliers for CE use to be able to grow further. The only way to increase the use of a technique comes through general availability.
For the firm implementation of a (or any) valuable tool in the pharmaceutical analysis toolbox, we need well-educated scientists and technicians. That naturally includes tools such as experimental design or chemometrics, quality by design, and statistics. Proper training is required not only on the science of the (new) technique, but also on good working practices and the requirements on highly-regulated pharmaceutical analysis. Markus Blümel of Novartis underlined this in his presentation at MSB 2012 in Geneva. CE-SDS methodology was implemented in R&D quality control in his company. Initially, more than 50% of the sequences run showed issues that could be anything from spikes, ghost peaks, tailing peaks, shifting migration times, or blocked capillaries. The method standard operating procedures (SOPs) were then updated with attention paid to the details of good working practice such as cleaning, capillary cutting, and rinsing, and the analysts were retrained on these practices. As a result, now more than 95% of the sequences run without issues (39). A decade ago, scientists in industry were still allowed time to implement new technology and take time to develop a better and faster analytical toolbox in the long run. Nowadays, the balance seems to have tipped over to short-term project deadlines, which impedes the implementation of new technologies. Yet, someone needs to fill the gap between theory and practice.
Concluding Remarks
CE techniques have huge potential, and their use in industry is increasing. Many routine, stable, robust, and sensitive CE methods are successfully being used in pharmaceutical and biotech analysis. There are several authority-approved applications, but there should and could be more. This is certainly achievable if we realize that a good method is something more than a good separation.
To achieve that, we need properly trained staff in all areas, that is, science, good laboratory practices (GLP), good manufacturing practices (GMP), and good working CE practices. The triangle (academia–vendors–industry) needs to work closely together to ensure that there are no gaps in knowledge transfer. Furthermore, additional instrument and application development is needed, as well as more competition to stimulate this. The way into the pharmacopeias is through nonproprietary methods, collaborative studies, and open discussions. The CE user network is paramount for sharing experience and good working practice, for as Einstein already noted, "In theory, theory and practice are the same. In practice, they are not."
References
(1) A. Tiselius, Trans. Faraday Soc. 33, 524–531 (1937).
(2) http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1948/index.html.
(3) S. Hjertén, Chromatogr. Rev. 9, 122–219 (1967).
(4) http://casss.org/displaycommon.cfm?an=1&subarticlenbr=562.
(5) R. Virtanen, Acta Polytech. Scand. 123, 1 (1974).
(6) F.E.P. Mikkers, F.M. Everaerts, and T.P.E.M. Verheggen, J. Chromatogr 169, 11 (1979).
(7) J.W. Jorgensen and K.D. Lukacs, Anal. Chem. 53, 1298 (1981).
(8) C.E. Sänger-van de Griend, "General Considerations to Improve Performance of CE Methods," in Capillary Electrophoresis Methods for Pharmaceutical Analysis, Separation Science and Technology Vol 9, S. Ahuja and M.I. Jimidar, Eds. (Academic Press, 2008).
(9) C.A. Lucy, A.M. MacDonald, and M.D. Gulcev, J. Chromatogr. A 1184, 81–105 (2008).
(10) C.E. Sänger-van de Griend and K. Gröningsson, J. Pharm. Biomed. Anal. 14, 295–304 (1996).
(11) C.E. Sänger-van de Griend, H Wahlström, K Gröningsson, and M Widahl-Näsman, J. Pharm. Biomed. Anal. 15, 1051–1061 (1997).
(12) http://www.sepscience.com/emails/CESol3eu.pdf.
(13) P.G. Muijselaar, "Overview of Current Regulatory Guidance," in Capillary Electrophoresis Methods for Pharmaceutical Analysis, Separation Science and Technology Vol 9, S. Ahuja and M.I. Jimidar, Eds. (Academic Press, 2008).
(14) U. Holzgrabe, "The Need for CE Methods in Pharmacopeial Monographs," in Capillary Electrophoresis Methods for Pharmaceutical Analysis, Separation Science and Technology Vol 9, S. Ahuja and M.I. Jimidar, Eds. (Academic Press, 2008).
(15) K.D. Altria, R.C. Harden, M. Hart, J. Hevizi, P.A. Hailey, J.V. Makwana, and M.J. Portsmouth, J. Chromatogr. A 641 147–153 (1993).
(16) K.D. Altria, N.G. Clayton, M. Hart, R.C. Harden, J. Hevizi, J.V. Makwana, and M.J. Portsmouth, Chromatographia 39, 180–184 (1994).
(17) K.D. Altria, N.G. Clayton, R.C. Harden, J.V. Makwana, and M.J. Portsmouth, Chromatographia 40, 47–50 (1995)
(18) E. Charton, J.H. McB Miller, F. Briançon, and G. Rautmann, Pharmeuropa Bio 1, 47 (2004).
(19) B. Nunnally, S.S. Park, K. Patel, M. Hong, X. Zhang, S-X. Wang, B. Rener, A. Reed-Bogan, O. Salas-Solano, W. Lau, M. Girard, H. Carnegie, V. Garcia-Canas, K.C. Cheng, M. Zeng M. Ruesch, R. Frazier, C. Jochheim, K. Natarajan, K. Jessop, M. Saeed, F. Moffatt, S. Madren, S. Thiam, and K. Altria, Chromatographia 64, 359–368 (2006).
(20) O. Salas-Solano, K. Babu, S.S. Park, X. Zhang, L. Zhang, Z. Sosic, B. Boumajny, M. Zeng, K.C. Cheng, A. Reed-Bogan, S. Cummins-Bitz, D.A. Michels, M. Parker, P. Bonasia, M. Hong, S. Cook, M. Ruesch, D. Lamb, D. Bolyan, S. Kiessig, D. Allender, and B. Nunnally, Chromatographia 73, 1137–1144 (2011).
(21) O. Salas-Solano, oral presentation about inter-company study on icIEF at CEPharm 2011.
(22) F. de l'Escaille and J.B. Falmagne, "Ion Analysis Using Capillary Electrophoresis," in Capillary Electrophoresis Methods for Pharmaceutical Analysis, Separation Science and Technology Vol 9, S. Ahuja and M.I. Jimidar, Eds. (Academic Press, 2008).
(23) O. Stålberg, K. Sander, and C.E. Sänger-van de Griend, J. Chromatogr. A 977, 265–275 (2002).
(24) Y. He, C. Isele, W. Hou, and M. Ruesch, J. Sep. Sci. 34, 548–555 (2011).
(25) B. Moritz, oral presentation at MSB 2012, Geneva.
(26) Pharmeuropa 11(2), 415 (1999).
(27) A. Bayol, A. Bristow, E. Charton, M. Girard, and P. Jongen, Pharmeuropa Bio. 1, 35 (2004).
(28) K. Persson-Stubberud and O. Åström, J. Chromatogr. A 798, 307–314 (1998).
(29) K. Persson-Stubberud and O. Åström, J. Chromatogr. A 826, 95–102 (1998).
(30) E. McEvoy, S. Donegan, J. Power, and K. Altria, Chromatographia 68, 49–56 (2008).
(31) C.E. Sänger-van de Griend, K. Gröningsson, and T. Arvidsson, J. Chromatogr. A 782 271–279 (1997).
(32) C.E. Sänger-van de Griend, Enantiomeric Separations by Capillary Electrophoresis in Pharmaceutical Analysis (Acta Universitatis Upsaliensis, 1999).
(33) M.M. Dittmann, K. Masuch, and G.P. Rozing, J. Chromatogr. A 887, 209 (2000).
(34) C. Karlsson, H. Wikström, D.W. Armstrong, and P.K. Owens, J. Chromatogr. A 897, 349 (2000).
(35) H. Wan, Drug Discovery Today 2, 171 (2005).
(36) W.H. Holmén, A.G. Wang, Y. Lindberg, W. Englund, M.B. Någård, and R.A. Thompson, Rapid Commun. Mass Spectrom. 17, 2639–2648 (2003).
(37) D. Visky, M.I. Jimidar, W. van Ael, T. Vennekens, D. Redlich, and M. de Smet, Electrophoresis 26, 1541–1549 (2005).
(38) C.D. Whitmore and L.A. Gennaro, Electrophoresis 33, 1550–1556 (2012).
(39) M. Blümel, oral presentation at MSB 2012, Geneva, Switzerland.
(40) D.K. Lloyd and H. Watzig, J. Chromatogr. B 663, 400–405 (1995).
This month's guest author:
Cari Sänger-van de Griend is a consultant and trainer at Kantisto BV, a pharmaceutical analytical consultancy she founded after 20 years in industry. She is a globally recognized expert on the implementation of CE techniques and is also a specialist on dissolution. She developed her lecturing and coaching skills during her years at Uppsala University in Sweden, Leiden University in The Netherlands, TNO, AstraZeneca, Solvay Pharmaceuticals, and Abbott.

Cari Sänger-van de Griend
The editor of Column Watch:
Ronald E. Majors "Column Watch" Editor Ronald E. Majors is a Senior Scientist in the Columns and Supplies Division at Agilent Technologies (Wilmington, Delaware), and is a member of LCGC's editorial advisory board. Direct correspondence about this column to lcgcedit@lcgcmag.com.

Ronald E. Majors

Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











